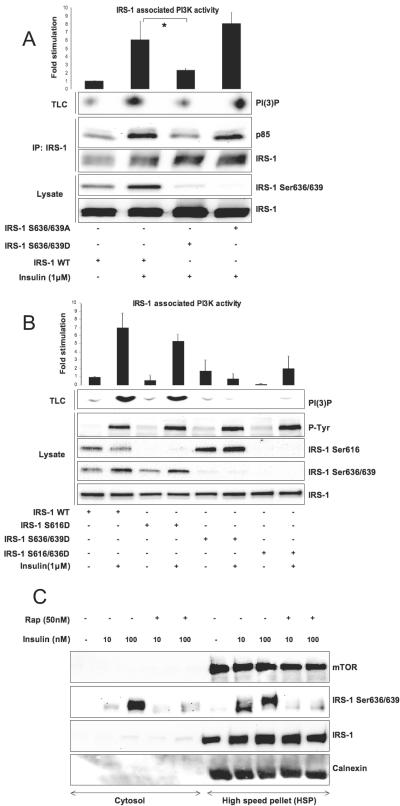
FIG. 2.
Phosphorylation of IRS-1 on Ser 636/639 but not on Ser616 uncouples insulin signaling. (A) 293HEK cells transiently transfected with specified IRS-1 mutants (4 μg of each cDNA per 10-cm plate) were serum starved for 6 h followed by 5 min of insulin stimulation and immunoprecipitation (IP) of IRS-1. The presence of PI3-kinase in the immunoprecipitated material was determined by Western blotting, and its activity was measured by TLC. The top panel shows normalized PI3-kinase activity (mean values ± SEM) of three independent experiments. The bottom panel shows Western blot analysis of total cell lysates. (B) Same as described for panel A with different IRS-1 mutants. Note that in panels A and B, phosphospecific antibodies do not recognize mutated serine residues in IRS-1, verifying that mutations have been introduced correctly. (C) L6 myotubes were homogenized and fractionated as described in Materials and Methods into cytosol and high-speed pellet. Each fraction was analyzed by Western blotting. The same amount of protein (30 μg) was loaded on each lane; asterisks indicate P values of < 0.01. +, present; −, absent.