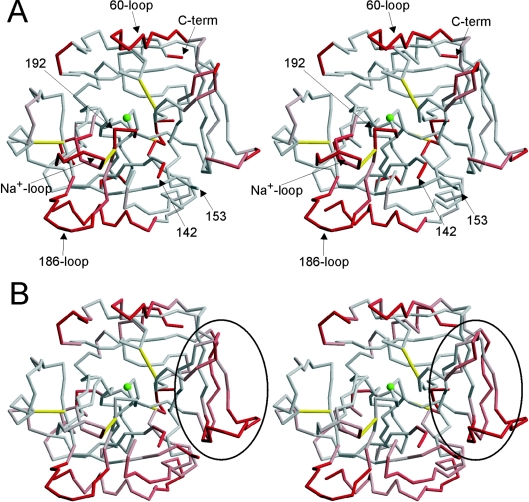
Figure 1. Structural comparison of Na+-free wild-type thrombin with fast thrombin.
(A) The stereo representation of the Cα trace of monomer AB coloured according to R.M.S.D. with thrombin in its normal fast conformation (1HAH) reveals an overall conserved fold, but with significant conformational changes in certain regions. The colour ramp is from 1 to 3 Å R.M.S.D., from grey to red. Thrombin is shown in the standard orientation, with disulphide bonds in yellow, significant regions labelled, and the active-site Ser195 represented as a green ball. (B) Stereo representation of the Cα trace of monomer AB (in the same orientation as above, with the green ball representing Ser195, and yellow rods indicating disulphide bonds), coloured according to B-factor, from grey to red for B-factors from 20 to 40 Å2. Exosite I is indicated by the oval.