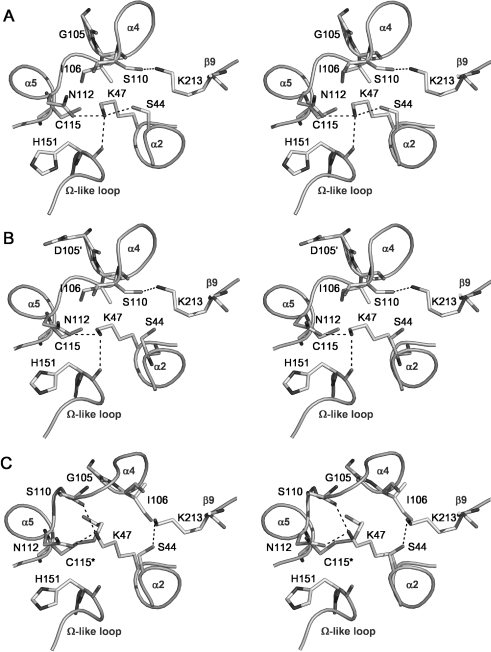
Figure 3. Hydrogen-bonding networks in the active sites of (A) wild-type PBP 5, (B) PBP 5-G105D (PBP 5′) and (C) Cys115 (2ME)-modified PBP 5, shown as stereo representations with important residues in bond form.
Potential hydrogen bonds are shown as dashed lines. In comparison with the views of wild-type and G105D mutant PBP 5, the Cys115 modification in the 2ME-modified enzyme has caused Ser110 to be displaced away from the active site and, as a result, its hydrogen bond with Lys213 is lost and Lys213 now contacts Ser44. Note also the altered position of Lys47 in the modified enzyme, which now contacts the carbonyl of Ser110 instead of His151. This Figure was prepared using PYMOL (pymol.sourceforge.net).