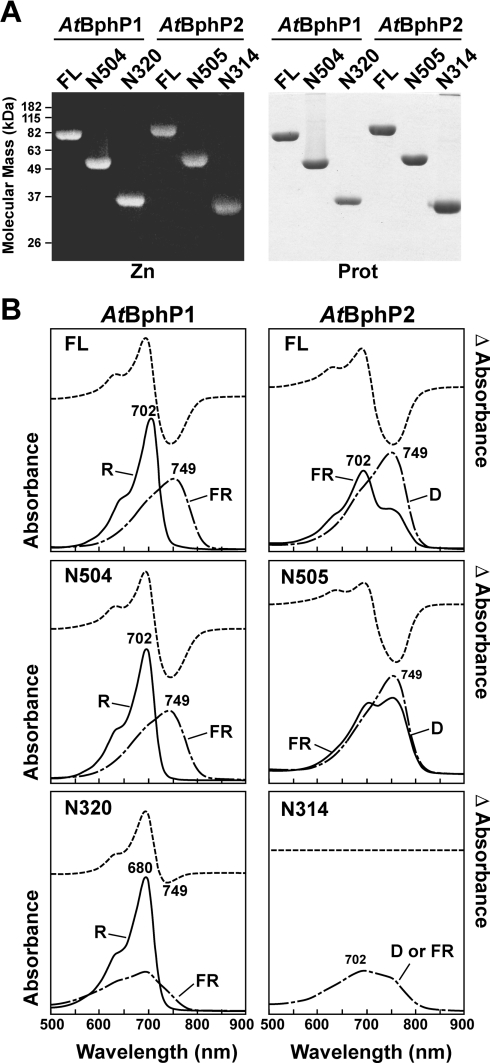
Figure 5. Assembly and spectral properties of full-length (FL) and C-terminally truncated forms of Ag. tumefaciens BphP1 and BphP2.
The numbers indicate the last amino acid of the truncated polypeptides. (A) Covalent binding of BV IXα. The wild-type (WT) and mutant apoproteins were incubated with BV IXα for 1 h, purified by nickel-chelate affinity chromatography and then subjected to SDS/PAGE and either assayed for the bound bilin by zinc-induced fluorescence (Zn) or stained for protein with Coomassie Blue (Prot). (B) Absorption and R/FR (AtBphP1) or FR/D (AtBphP2) difference spectra of samples from (A). The absorption maxima of the Pr and Pfr forms are indicated. The spectra were adjusted for an equal amount of protein as determined by absorbance at 280 nm.