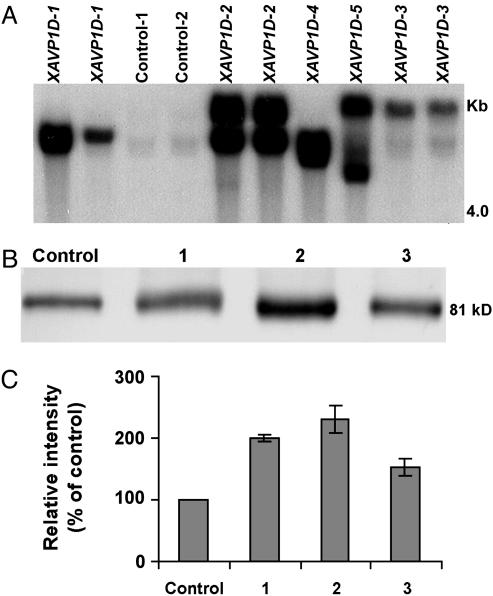
Fig. 1.
Molecular characterization of AVP1D expression in transgenic tomato. (A) Southern blot analysis indicates the absence of the transgene from control plants, and the presence of the 35S::AVP1D construct in genomic DNA of the transgenic tomato plants. In all cases, genomic DNA (10 μg) was digested with EcoRI, separated on a 0.9% agarose gel by electrophoresis, and probed with a BglII fragment of the AVP1D ORF. Results are shown for two replicate plants for each of the controls, and five independently generated transgenic lines (XAVP1D-1, -2, -3, -4, and -5). (B) Western blot analysis of H+-PPase protein expression in control and transgenic tomato. Size-fractionated protein (20 μg protein per lane) from tonoplast-enriched vesicles prepared from root tissue of vector-transformed (control) and XAVP1D-1 (blot 1), -2 (blot 2), and -3 (blot 3) lines was probed with an antibody immunoreactive to both the endogenous, and the recombinant H+-PPase. Results are shown for one representative experiment with a control plant and three transgenic lines (see A); this experiment was repeated a total of four times. Migration of molecular mass markers indicated that the single immunoreactive band in each lane had a mass of ≈81 kDa (i.e., the deduced mass of the endogenous and recombinant H+-PPases). (C) Quantification of relative H+-PPase protein levels in control (Control) and transgenic plants (lines 1–3 as above). Pooled results (means ± SE) are presented for four experiments similar to that shown in B; data are presented as protein levels in transgenic lines relative to that found in the control plants.