Abstract
Human chromosome 22q11.2 has been implicated in various behavioral abnormalities, including schizophrenia and other neuropsychiatric/behavioral disorders. However, the specific genes within 22q11.2 that contribute to these disorders are still poorly understood. Here, we show that an ≈200-kb segment of human 22q11.2 causes specific behavioral abnormalities in mice. Mice that overexpress an ≈200-kb region of human 22q11.2, containing CDCrel, GP1Bβ, TBX1, and WDR14, exhibited spontaneous sensitization of hyperactivity and a lack of habituation. These effects were ameliorated by antipsychotic drugs. The transgenic mice were also impaired in nesting behavior. Although Tbx1 has been shown to be responsible for many physical defects associated with 22q11.2 haploinsufficiency, Tbx1 heterozygous mice did not display these behavioral abnormalities. Our results show that the ≈200-kb region of 22q11.2 contains a gene(s) responsible for behavioral abnormalities and suggest that distinct genetic components within 22q11.2 mediate physical and behavioral abnormalities.
Keywords: 22q11, habituation, hyperactivity, mouse model, schizophrenia
The genetic abnormality in 22q11.2 is better delineated than those of most human chromosomal loci that have been implicated in neuropsychiatric and behavioral disorders. Higher-than-expected rates of schizophrenia and other neuropsychiatric disorders are seen in adult patients with 1.5- to 3-Mb haploinsufficiency (i.e., deletion) in 22q11.2 (1-7). Reciprocal duplication of the same chromosomal region also has been identified, and these individuals exhibit impulsivity, aggression, oppositional defiant disorder, social immaturity, short attention spans, attention deficit disorder, and cognitive deficits (8-12). Because most of these individuals are infants or children at present, their neuropsychiatric disorders have not been fully characterized. However, preliminary data show that individuals with duplications also suffer from neuropsychiatric disorders (9, 12).
It remains poorly understood precisely how a gene or set of genes within 22q11.2 contributes to these behavioral phenotypes. In non-duplication/deletion cases, polymorphisms of various genes residing in 22q11.2 have been associated with schizophrenia and related behavioral and cognitive defects (13, 14). For example, a high activity allele of catechol-O-methyl-transferase (COMT) is associated with a heightened risk for schizophrenia in humans (15). As for 22q11.2 deletion cases, haploinsufficiency is associated with prepulse inhibition (PPI) deficits in humans (16), and a comparable deficit is seen in mice with a relatively large 1-Mb heterozygosity, including 19 genes of the mouse synteny of human 22q11.2 (17). Mice defective for either Prodh (proline dehydrogenase) or Zdhhc8 (zinc finger, DHHC-type containing 8) exhibit much weaker PPI deficits (18, 19). Given the presumed polygenetic nature of neuropsychiatric disorders and the weak PPI deficits observed in Prodh- and Zdhhc8-deficient mice, it is likely that other 22q11.2 genes contribute to behavioral abnormalities.
Another critical issue is whether the same set of genes contributes to the behavioral and physical abnormalities observed in response to 22q11.2 deletion and duplication. Patients with 22q11.2 deletions and duplications also show physical abnormalities encompassing the cardiovascular, velophargyngeal, and ear systems. Tbx1 (T-box 1), a 22q11.2 gene encoding a T-box transcription factor, is responsible, at least in part, for most of these physical defects (20-29), but it is not known whether this gene also mediates behavioral abnormalities.
We have identified an ≈200-kb region of human 22q11.2, which includes CDCrel (cell division control-related protein 1), GP1Bβ (glycoprotein Ib, β polypeptide), TBX1, and WDR14, that, when overexpressed in mice, is associated with sensitized hyperactivity and lack of habituation. These effects are reversed when mice are treated with antipsychotic drugs. The transgenic (TG) mice also exhibited defects in nesting behavior. Although heterozygosity of Tbx1 is associated with many physical abnormalities in mice (20-22, 24, 26-28), Tbx1 heterozygous mice did not exhibit such behavioral abnormalities. Our data suggest that this ≈200-kb region of 22q11.2 contains one or more genes that are responsible for some behavioral disorders associated with 22q11.2 duplications, and that heterozygosity of Tbx1 alone is not responsible for all behavioral abnormalities in 22q11.2 deletion cases.
Materials and Methods
Mice. Transgenic mice carrying 8-10 copies (BAC316.23, coisogenic FVB background) or 1-2 copies (BAC316.27, a mixed C57BL/6J and FVB background, N4 in FVB) of a bacterial artificial chromosome (BAC), harboring human CDCrel, GP1Bβ, TBX1, and WDR14, were generated, as described (22, 30). These two lines of BAC TG mice were separately developed by injecting the BAC into different pronuclei, resulting in different copy numbers and presumably different sites of insertion in the mouse genome. All four transgenes, as well as their mouse orthologs, are expressed in the brain (30). Male and female mice were tested at the age of 2-4 months. A separate group of adolescent BAC316.23 mice were tested at the age of 5 weeks.
Male and female congenic Tbx1 heterozygous mice and their WT littermates were tested at the age of 2-4 months. These mice are heterozygous for Tbx1 only and do not express the BAC transgene. This mouse line was originally developed in a mixed genetic background of 129svJ and C57BL/6J, and was later backcrossed to FVB mice for 12 generations (22). Tbx1 homozygous mice were not used for behavioral analysis, because they were not born alive (20-22)
Mice were housed on a 14-h light/10-h dark cycle with access to food and water ad libitum. All experimental procedures were approved by the Animal Institute Committee of the Albert Einstein College of Medicine.
Behavioral Analyses. Spontaneous locomotor activity. High copy (316.23) and low copy (316.27) BAC TG mice and their WT littermates or Tbx1 heterozygous mice and their WT littermates were tested in open field activity apparatuses (Truscan, Coulbourn Instruments, Lehigh Valley, PA). Horizontal activity was recorded for 30 min per day, and distance traveled was used as an index of horizontal activity (31).
The acute effects of haloperidol and clozapine on hyperactivity were tested in adult BAC (316.23) TG mice and their WT littermates. Locomotor activity was measured for 8 days. Haloperidol (0, 0.5, or 1.0 mg base/kg, s.c., Sigma) or clozapine (0, 10, or 20 mg base/kg, s.c., Sigma and a gift from Novartis) was injected 30 min before the 8th session.
We devised a chronic injection regimen in BAC (316.23) TG mice to avoid the acute motor effects of clozapine while maintaining the chronic effects. A separate set of adult BAC TG and WT littermates received clozapine injections (0, 5, or 10 mg base/kg, s.c.) for 3 weeks in their home cages before behavioral testing began. This duration of antipsychotic treatment is required to restore sensorimotor gating deficits in rodents (32) and to significantly attenuate schizophrenic symptoms in humans (33). During testing, mice were injected with either vehicle or clozapine 1-2 h after each testing session, to avoid the acute motor effects of clozapine on behavior while maintaining chronic treatment. Mice were tested in an open field for 7 days. For qualitative analysis of movement paths, mice were tested for 5 min on either day 8 or 9.
The half-life of clozapine in mice is 110 min, and it is completely cleared from the rodent brain within 12 h (34). Therefore, no remaining clozapine would be expected to accumulate between sessions. Because the behavioral effects of these drugs within the selected dose range disappear within 6 h in rodents (35), the motor depressant effects of clozapine would not be expected to carry over into the next day.
Amphetamine test. A separate group of Tbx1 mice was tested for the locomotor activating effects of d-amphetamine (0, 0.5, or 1.0 mg base/kg, s.c.). Tbx1 heterozygous mice and their WT littermates received saline injections for the first 3 days and amphetamine on the 4th day. Tbx1 heterozygous mice and WT littermates showed a stable baseline level of spontaneous locomotor activity by day 4 of habituation. Mice received injections immediately before being placed in an open field.
Nest building. We followed a published procedure for evaluating nesting behavior (36-38). Adult BAC (316.23) mice were singly housed in individual cages with food and water available ad libitum. A weighed cotton nestlet (5 × 5 cm, Ancare, Bellmore, NY) was placed on the wire cage lid. The cotton remaining on the lid was weighed on each of 3 consecutive days. The depth of a nest and the amount of a nestlet used to build a nest were measured daily by an observer blinded to genotype. After each measurement, the nest within the cage was removed daily and a new nestlet was placed on the cage lid. The temperature of the animal room was held constant at 20°C-22°C, because mice tend to build bigger nests in a cold room (36). Rectal temperature was recorded by using a digital thermometer.
Statistical Analyses. All quantitative data were analyzed by ANOVA. When significance was detected, Newman-Keuls's post hoc tests were used for further analysis. When there were only two groups for comparison, a t test was used.
Results and Discussion
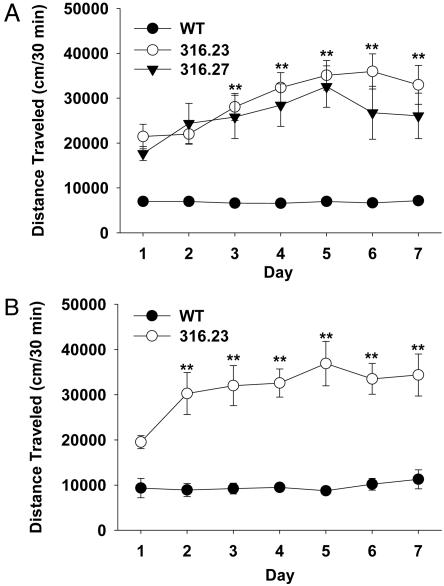
Overexpression of Four Human 22q11.2 Genes Induces Hyperactivity and Sensitization. High copy (316.23) and low copy (316.27) BAC TG mice displayed equal levels of hyperactivity that became progressively sensitized in the absence of pharmacological stimulation at the age of 2-4 months (Fig. 1A). Adolescent BAC TG mice (316.23) also showed hyperactivity and spontaneous sensitization at the age of 5 weeks (Fig. 1B). Because the two separate lines of TG mice with different genetic backgrounds exhibit indistinguishable abnormalities in locomotor activity, the behavioral abnormalities are unlikely to result from an accidental disruption by the BAC of an endogenous mouse gene or from genetic backgrounds.
Fig. 1.
BAC TG mice exhibited hyperactivity and its spontaneous sensitization. Mice were tested in an open field for 30 min on 7 consecutive days. (A) Two separate adult BAC TG mouse lines overexpressed 8-10 copies of the BAC (316.23) or 1-2 copies of the BAC (316.27). Both lines of BAC TG mice showed hyperactivity (F2,59 = 41.9, P < 0.01). TG mice, but not WT mice, showed spontaneous sensitization over 7 days as evidenced by interaction between genotype and day (F12,354 = 4.5, P < 0.01). WT, n = 34; BAC316.23, n = 19; BAC316.27, n = 9. (B) Five-week-old, adolescent WT and TG mice (316.23) differed (genotype, F1,16 = 61.44, P < 0.01), and TG, but not WT, mice showed sensitization of locomotor activity, as evidenced by a significant interaction between genotype and day (F6,96 = 3.44, P < 0.01). WT, n = 10; TG, n = 8. **, Significant difference for the two TG mouse lines from day 1 at 1% level (Newman-Keuls post hoc tests). SEMs were relatively small in WT mice, and the error bars are not visible at this scale.
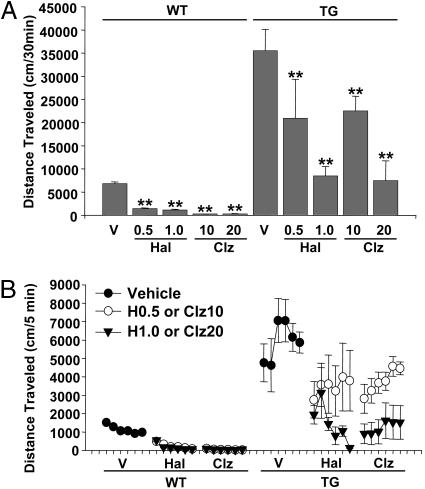
Antipsychotic Drugs Attenuate Hyperactivity and Reinstate Habituation in BAC Transgenic Mice. Although it is inherently difficult to model the symptoms of human neuropsychiatric conditions in mice, an approach to ascertain the correlation of this mouse behavior with one aspect of schizophrenia is to test whether antipsychotic drugs ameliorate the behavioral abnormality (i.e., predictive validity). Antipsychotic drugs attenuate schizophrenic symptoms in humans with or without 22q11.2 abnormality (39-41). Therefore, we tested whether the sensitized locomotor hyperactivity exhibited by the BAC (316.23) TG mice responds to acute treatments with the two antipsychotic drugs clozapine and haloperidol. Clozapine and haloperidol acutely reduced the sensitized locomotor activity in BAC mice on day 8 (Fig. 2). The clinically relevant doses of clozapine and haloperidol in rodents are estimated to be 5-15 mg/kg and 0.04-0.08 mg/kg, respectively (42, 43). Although clozapine attenuated hyperactivity within this dose range, similar levels of reduction in hyperactivity were seen at high doses of haloperidol.
Fig. 2.
Acute effects of antipsychotics on sensitized hyperactivity in BAC TG mice (316.23). Mice were tested in the open field for 8 days and received acute injections of vehicle (V), haloperidol (Hal; 0.5, 0.5 mg/kg; 1.0, 1.0 mg/kg; s.c.) or clozapine (Clz; 10, 10 mg/kg; 20, 20 mg/kg; s.c.) 30 min before testing on day 8. Horizontal locomotor activity was recorded for 30 min. (A) TG and WT mice differed (F1,91 = 190.03, P < 0.01) and the drugs significantly attenuated locomotor activity (F4,91 = 25.09, P < 0.01). The drugs reduced locomotor activity to different degrees in WT and TG mice, as evidenced by a significant interaction between genotype and treatment (F4,91 = 11.82, P < 0.01). Asterisks indicate a statistically significant difference from respective vehicle controls at 1% (**) levels, as determined by Newman-Keuls tests. Error bars represent ±SEM. n = 5-21 mice per group. (B) Locomotor activity at every 5 min for a 30-min session. The drugs differentially reduced locomotor activity during the session in WT and TG mice (genotype × drug × time, F20,455 = 20.02, P < 0.01). Both drugs reduced spontaneous locomotor activity in WT mice (F4,71 = 109.81, P < 0.01) significantly more during later time points (drug × time, F20,355 = 5.07, P < 0.01). The drugs reduced the sensitized hyperactivity in TG mice (F4,20 = 5.33, P < 0.01) in a time-dependent manner (drug × time, F20,100 = 2.20, P < 0.01). Error bars represent ±SEM. H0.5, Haloperidol 0.5 mg/kg; H1, haloperidol, 1.0 mg/kg; Clz10, clozapine 10 mg/kg; Clz20, clozapine 20 mg/kg.
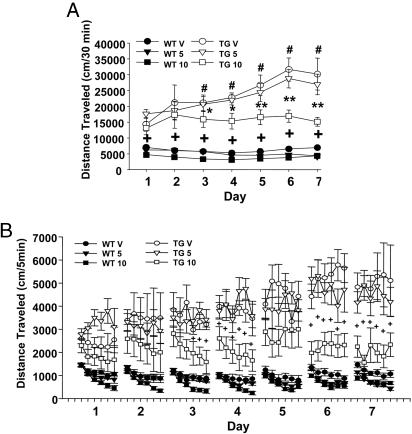
Clozapine does not generally induce extrapyramidal motor side effects in humans (39), but it acutely reduces spontaneous locomotor activity in mice (44-47). In fact, clozapine, as well as haloperidol, attenuated normal motor activity in WT mice, presumably due to their sedative or nonspecific motor depressant effects (see Fig. 2). We developed an injection schedule to maintain chronic treatment without inducing the acute sedative effects of clozapine. Mice were treated with clozapine for 3 weeks before the onset of behavioral analysis, and clozapine was injected 1-2 h after each test session during behavioral sessions. This treatment significantly attenuated the development of sensitization of hyperactivity in BAC mice without attenuating hyperactivity per se (Fig. 3A). Moreover, clozapine at least partially reinstated a habituation-like decline in hyperactivity in BAC TG mice like vehicle-treated WT mice [see Fig. 3B, clozapine (10 mg/kg) treatment BAC group]. Locomotor activity in clozapine-treated BAC TG mice was gradually reduced within each of the first four sessions, and low levels of activity were maintained on days 6 and 7. By contrast, a habituation-like decline was not seen in TG mice after acute clozapine treatment (see Fig. 2B).
Fig. 3.
Chronic clozapine treatment blocks the sensitization of hyperactivity in BAC TG mice. WT and TG (316.23) mice were treated with either vehicle (V) or clozapine (5 or 10 mg/kg, s.c.) for 3 weeks in their home cages and subsequently tested in an open field for 7 days. During testing, vehicle or clozapine was given 1-2 h after each 30-min testing session, to avoid the acute effects of clozapine but to maintain chronic treatment. Data are presented for 7 days (A) and at every 5 min for each 30-min session (B). (A) TG mice differed from WT mice (F1,39 = 232.65, P < 0.01) in their response to drugs (genotype × treatment, F2,39 = 8.96, P < 0.01) and over days (genotype × day, F6,234 = 12.83, P < 0.01). The three-way interaction was significant (genotype × treatment × day, F12,234 = 2.4, P < 0.01). Note that clozapine blocked the development of sensitized locomotor activity without reducing hyperactivity per se. #, A statistically significant difference from Day 1 in vehicle-treated TG mice. * and **, A statistically significant difference between vehicle-treated and clozapine (10 mg/kg)-treated TG mice at 5% and 1% levels, respectively. +, A statistically significant difference in all dose groups between WT and TG mice at 1% level (Newman-Keuls post hoc test). (B) Chronic clozapine exerted differential effects on WT and TG mice over 7 days, as evidenced by significant three-way interaction among genotype, treatment, and day (F2,141 = 9.78, P < 0.01). Clozapine dose-dependently attenuated locomotor sensitization in TG mice across sessions (dose × session, F12,108 = 2.2, P < 0.05) and within sessions (dose × time intervals, F60,540 = 1.41, P < 0.05). +, A statistically significant difference between vehicle-treated and clozapine (10 mg/kg)-treated TG mice (P < 0.05, Newman-Keuls post hoc tests). Error bars represent ±SEM. n = 6-9 mice per group.
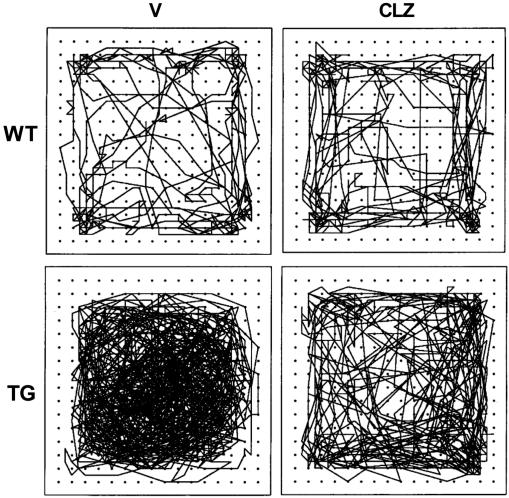
The compulsive nature of sensitized hyperactivity was apparent in the movement paths of vehicle-treated BAC TG mice, as compared with random paths of WT mice (Fig. 4; see also Movie 1, which is published as supporting information on the PNAS web site). Chronic clozapine treatment significantly attenuated the compulsive, sensitized movement pattern exhibited by BAC TG mice. This behavioral pattern also demonstrates that the reduced hyperactivity seen in clozapine-treated BAC TG mice is not due to increased focal stereotyped behavior, which would have replaced and reduced hyperactivity. The level of hyperactivity was so high that no normal inter-individual interaction was possible between a TG mouse and a WT mouse (see Movie 2, which is published as supporting information on the PNAS web site).
Fig. 4.
Representative movement paths of mice. The movement path of mice chronically treated with vehicle (V) or clozapine (CLZ, 10 mg/kg, s.c., 3 weeks followed by 1-2 h after each session) was recorded for 5 min on the 8th day; longer testing yielded movement paths too dense to discern. Some mice were tested on the 9th day and showed the same pattern (data not shown). WT, wild-type littermates; TG, BAC TG mice (316.23). n = 6-9 mice per group.
Both lines of BAC mice (317.23 and 317.27) exhibit abnormalities in the vestibular and middle/inner ear systems and circling behavior (26, 30). However, it is unlikely that the hyperactivity we detected is entirely due to circling behavior. BAC mice clearly show noncircling hyperactivity (see Movies 1 and 2), and the two antipsychotic drugs with no known action on the vestibular/ear system significantly attenuated the sensitized hyperactivity (Fig. 2 and 3). Moreover, although low-copy BAC mice (317.27) show fewer vestibular deficits than high-copy BAC mice (317.23) (30), the two lines of mice showed indistinguishable levels of hyperactivity and sensitization (see Fig. 1). Thus, although we cannot exclude the possibility that circling behavior contributes to the hyperactivity, the BAC mice also exhibit noncircling hyperactivity that is independent of vestibular deficits.
BAC Transgenic Mice Are Impaired in Nesting. Nest building is considered to be an expression of natural mouse behavior that allows mice to camouflage themselves from predators in the wild (48). This behavior is sensitive to disruption of non-22q11 genes implicated in schizophrenia (38, 49). BAC TG mice did not make nests or made shallower nests than those of WT littermates (Table 1). It is unlikely that this behavioral defect simply reflects the extraordinarily high level of hyperactivity, because BAC TG mice often exhibit normal motor activity in their home cage and are capable of eating food pellets from the cage ceiling (data not shown). Moreover, hyperactivity per se does not necessarily cause less nest-building activity in mice (50). It is also unlikely that hyperactivity increased body temperature in TG mice, which indirectly reduced the size of nests, because WT and TG mice have indistinguishable levels of body temperature [WT, 37.2°C (SEM ± 0.26); TG, 37.1°C (SEM ± 0.31); t(14) = 0.31, not significant (n.s.)].
Table 1. Nest depths of WT and TG mice.
| Nest depth, cm
|
|||
|---|---|---|---|
| Mice | Day 1 | Day 2 | Day 3 |
| WT (n = 23) | 3.8 ± 0.2 | 3.8 ± 0.2 | 4.2 ± 0.3 |
| TG (n = 8) | 0.1 ± 0.04** | 0.5 ± 0.4** | 0.1 ± 0.1** |
Mean ± SEM of nest depths. WT and BAC TG (316.23) mice differed (F1,29 = 117.1, P < 0.01) without daily fluctuation (F2,58 = 0.43, n.s.) or interaction (F2, 58 = 1.1, n.s.). **, Significance between WT and TG mice (P < 0.01) was determined by Newman-Keul's post hoc tests.
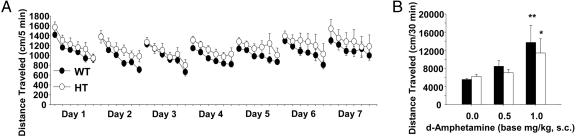
Tbx1 Heterozygous Mice Are Normal in Locomotor Activity, Habituation, Nesting, and Locomotor Response to Amphetamine. Mice with heterozygous deletion or overexpression of Tbx1 have been shown to exhibit cardiovascular and middle/inner ear defects associated with human 22q11.2 haploinsufficiency and duplication cases (20-22, 24, 26-28). In non-deletion/duplication cases, Tbx1 mutation with reduced transcriptional activity is also associated with cardiovascular abnormalities in humans (51, 52). Therefore, we examined whether heterozygosity of this gene alone causes behavioral abnormalities. Tbx1 heterozygous mice exhibited normal levels of spontaneous locomotor activity and daily habituation (Fig. 5A) and nest building behavior (Table 2).
Fig. 5.
Spontaneous locomotor activity and amphetamine-induced locomotor activity in Tbx1 heterozygous (HT) mice. (A) Time course of locomotor activity in Tbx1 HT mice and WT littermates. Data are presented at every 5 min for each 30-min session. Both HT and WT mice showed a daily habituation (F6,150 = 6.14, P < 0.01), but locomotor activity did not differ between the two groups (F1,25 = 1.30, n.s.). Error bars represent ±SEM. WT, n = 14; HT, n = 13. (B) Effects of d-amphetamine on spontaneous locomotor activity in WT littermates (filled bars) and Tbx1 heterozygous mice (open bars). Mice were tested in the open field for 3 days and received acute injections of d-amphetamine (0, 0.5, or 1.0 mg/kg, s.c.) immediately before testing on day 4. Horizontal locomotor activity was recorded for 30 min. Data are presented for each 30-min session. Although the drug significantly increased locomotor activity in a dose-dependent manner (F2,56 = 8.69, P < 0.01), HT and WT mice did not differ (F1,56 = 0.58, n.s.). Asterisks indicate a statistically significant difference from respective vehicle controls at 5% (*) and 1% (**) levels, as determined by Newman-Keuls tests. Error bars represent ±SEM. n = 6-16 mice per group.
Table 2. Nest depths of WT and HT mice.
| Nest depth, cm
|
|||
|---|---|---|---|
| Mice | Day 1 | Day 2 | Day 3 |
| WT (n = 11) | 3.3 ± 0.3 | 3.2 ± 0.3 | 3.1 ± 0.2 |
| HT (n = 6) | 3.5 ± 0.6 | 3.4 ± 0.7 | 2.5 ± 0.9 |
Mean ± SEM of nest depths. WT and Tbxl HT mice did not differ (F1,15 = 0.06, n.s.). There was no daily fluctuation (F2, 30 = 1.1, n.s.) or interaction (F2, 30 = 0.68, n.s.). There was no significant difference between WT and HT mice, as determined by Newman-Keuls's post hoc tests. HT, Tbxl heterozygous mice.
Even when schizophrenic patients are asymptomatic during remission, psychomotor stimulants such as amphetamine can evoke behavioral effects in these patients at doses that have no discernible effect in normal individuals (53). To further examine the relevance of Tbx1 to behavioral abnormalities, we examined the behavioral response to amphetamine in Tbx1 heterozygous mice and WT littermates. The locomotor response to amphetamine was indistinguishable between heterozygous and WT mice (Fig. 5B). Given that Tbx1 deletion in mice, at least in part, recapitulates most of the physical abnormalities of human 22q11.2 haploinsufficiency (20-29), our results suggest that different genes are responsible for physical and behavioral abnormalities associated with 22q11.2.
Our study shows that overexpression of the ≈200-kb region of the human 22q11.2 is associated with the absence of habituation, antipsychotic-responsive sensitized hyperactivity, and abnormal nesting. The relevance of these mouse behavioral abnormalities to the human neuropsychiatric disorders associated with 22q11.2 duplication remains unclear. Because most individuals with 22q11.2 duplication who have been identified to date are infants or children, their adulthood neuropsychiatric profile has not been fully characterized. However, these children exhibit a host of developmental, behavioral, and cognitive defects (8-12) that are present among children who subsequently develop schizophrenia (54, 55). Moreover, preliminary data suggest that individuals with 22q11.2 duplication also suffer from neuropsychiatric disorders (9, 12).
Three aspects of the behavioral abnormalities of BAC TG mice are also consistent with some characteristics of schizophrenia. First, behavioral excitement is a salient feature of schizophrenia with 22q11.2 deletion (6). Hyperactivity is seen in mice lacking non-22q11 genes that have been implicated in schizophrenia (e.g., calcineurin, NMDA receptor, and neuregulin1) (38, 45, 56, 57). Moreover, hyperactivity is induced in rodents by psychotomimetics, which induce schizophrenic symptoms in humans (58). Similarly, nesting deficits have been observed in mice that lack calcineurin, a non-22q11 gene implicated in schizophrenia (38). Second, hyperactivity in BAC TG mice was attenuated by chronic clozapine injections, a treatment known to attenuate schizophrenic symptoms in patients with 22q11.2 deletions (40, 41). Third, chronic clozapine treatment partly restored a habituation-like decline in hyperactivity in BAC TG mice. The absence of habituation is cross-modally seen in schizophrenic patients and is thought to be an intermediate trait of schizophrenia (59).
It is important to point out that there is no animal model that recapitulates the entire symptomatology of schizophrenia; available animal models mimic only certain traits of schizophrenia (see Supporting Text, which is published as supporting information on the PNAS web site). Because there is no trait or symptom specific to schizophrenia (60), it is equally possible that these behavioral abnormalities of BAC TG mice are relevant to other neuropsychiatric/behavioral symptoms of patients with 22q11.2 duplications and deletions.
Although more work is needed to ascertain the role of single 22q11.2 genes and interaction among them in the etiology of behavioral abnormalities, this mouse model contributes to a better understanding of the 22q11.2 mechanisms underlying neuropsychiatric and behavioral disorders.
Supplementary Material
Acknowledgments
We thank Novartis for their generous gift of clozapine. We are grateful for support from the Department of Psychiatry and Behavioral Sciences and from the Program in Human Genetics/Howard Hughes Funds, Albert Einstein College of Medicine (to N.H.), National Institutes of Health Grant R01DC005186 (to B.M.), and the Bronx Psychiatric Center, the State of New York (to S.A.).
Author contributions: N.H. designed research; H.Z., M.L., T.S., and S.A. performed research; B.F., M.A., M.I., R.K., and B.M. contributed new reagents/analytic tools; N.H., M.L., M.A., M.I., and T.S. analyzed data; and N.H. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: Tbx1, T-box 1; BAC, bacterial artificial chromosome; TG, transgenic; n.s., not significant.
References
- 1.Shprintzen, R. J., Goldberg, R., Golding-Kushner, K. J. & Marion, R. W. (1992) Am. J. Med. Genet. 42, 141-142. [DOI] [PubMed] [Google Scholar]
- 2.Pulver, A. E., Nestadt, G., Goldberg, R., Shprintzen, R. J., Lamacz, M., Wolyniec, P. S., Morrow, B., Karayiorgou, M., Antonarakis, S. E., Housman, D., et al. (1994) J. Nerv. Ment. Dis. 182, 476-478. [DOI] [PubMed] [Google Scholar]
- 3.Papolos, D. F., Faedda, G. L., Veit, S., Goldberg, R., Morrow, B., Kucherlapati, R. & Shprintzen, R. J. (1996) Am. J. Psychiatry 153, 1541-1547. [DOI] [PubMed] [Google Scholar]
- 4.Carlson, C., Papolos, D., Pandita, R. K., Faedda, G. L., Veit, S., Goldberg, R., Shprintzen, R., Kucherlapati, R. & Morrow, B. (1997) Am. J. Hum. Genet. 60, 851-859. [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy, K. C., Jones, L. A. & Owen, M. J. (1999) Arch. Gen. Psychiatry 56, 940-945. [DOI] [PubMed] [Google Scholar]
- 6.Bassett, A. S., Chow, E. W., AbdelMalik, P., Gheorghiu, M., Husted, J. & Weksberg, R. (2003) Am. J. Psychiatry 60, 1580-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gothelf, D., Presburger, G., Zohar, A. H., Burg, M., Nahmani, A., Frydman, M., Shohat, M., Inbar, D., Aviram-Goldring, A., Yeshaya, J., et al. (2004) Am. J. Med. Genet. 126B, 99-105. [DOI] [PubMed] [Google Scholar]
- 8.Edelmann, L., Pandita, R. K., Spiteri, E., Funke, B., Goldberg, R., Palanisamy, N., Chaganti, R. S., Magenis, E., Shprintzen, R. J. & Morrow, B. E. (1999) Hum. Mol. Genet. 8, 1157-1167. [DOI] [PubMed] [Google Scholar]
- 9.Ensenauer, R. E., Adeyinka, A., Flynn, H. C., Michels, V. V., Lindor, N. M., Dawson, D. B., Thorland, E. C., Lorentz, C. P., Goldstein, J. L., McDonald, M. T., et al. (2003) Am. J. Hum. Genet. 73, 1027-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassed, S. J., Hopcus-Niccum, D., Zhang, L., Li, S. & Mulvihill, J. J. (2004) Clin. Genet. 65, 400-404. [DOI] [PubMed] [Google Scholar]
- 11.Yobb, T. M., Somerville, M. J., Willatt, L., Firth, H. V., Harrison, K., MacKenzie, J., Gallo, N., Morrow, B. E., Shaffer, L. G., Babcock, M., et al. (2005) Am. J. Hum. Genet. 76, 865-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoi, M. F., Lebas, F., Gruchy, N., Ardalan, A., Biran-Mucignat, V., Malan, V., Finkel, L., Roger, G., Ducrocq, S., Gold, F., et al. (2005) Am. J. Med. Genet. A 137, 47-51. [DOI] [PubMed] [Google Scholar]
- 13.Karayiorgou, M. & Gogos, J. A. (2004) Mol. Brain Res. 132, 95-104. [DOI] [PubMed] [Google Scholar]
- 14.Mowry, B. J., Holmans, P. A., Pulver, A. E., Gejman, P. V., Riley, B., Williams, N. M., Laurent, C., Schwab, S. G., Wildenauer, D. B., Bauche, S., et al. (2004) Mol. Psychiatry 9, 784-795. [DOI] [PubMed] [Google Scholar]
- 15.Glatt, S. J., Faraone, S. V., Tsuang & M. T. (2003) Am. J. Psychiatry 160, 469-476. [DOI] [PubMed] [Google Scholar]
- 16.Sobin, C., Kiley-Brabeck, K. & Karayiorgou, M. (2005) Am. J. Psychiatry 162, 1090-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paylor, R., McIlwain, K. L., McAninch, R., Nellis, A., Yuva-Paylor, L. A., Baldini, A. & Lindsay, E. A. (2001) Hum. Mol. Genet. 10, 2645-2650. [DOI] [PubMed] [Google Scholar]
- 18.Gogos, J. A., Santha, M., Takacs, Z., Beck, K. D., Luine, V., Lucas, L. R., Nadler, J. V. & Karayiorgou, M. (1999) Nat. Genet. 21, 434-439. [DOI] [PubMed] [Google Scholar]
- 19.Mukai, J., Liu, H., Burt, R. A., Swor, D. E., Lai, W. S., Karayiorgou, M. & Gogos, J. A. (2004) Nat. Genet. 36, 725-731. [DOI] [PubMed] [Google Scholar]
- 20.Jerome, L. A. & Papaioannou, V. E. (2001) Nat. Genet. 27, 286-291. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay, E. A., Vitelli, F., Su, H., Morishima, M., Huynh, T., Pramparo, T., Jurecic, V., Ogunrinu, G., Sutherland, H. F., Scambler, P. J., et al. (2001) Nature 410, 97-101. [DOI] [PubMed] [Google Scholar]
- 22.Merscher, S., Funke, B., Epstein, J. A., Heyer, J., Puech, A., Lu, M. M., Xavier, R. J., Demay, M. B., Russell, R. G., Factor, S., et al. (2001) Cell 104, 619-629. [DOI] [PubMed] [Google Scholar]
- 23.Vitelli, F., Viola, A., Morishima, M., Pramparo, T., Baldini, A. & Lindsay, E. (2003) Hum. Mol. Genet. 12, 2041-2048. [DOI] [PubMed] [Google Scholar]
- 24.Hu, T., Yamagishi, H., Maeda, J., McAnally, J., Yamagishi, C. & Srivastava, D. (2004) Development (Cambridge, U.K.) 131, 5491-5502. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, R. G., Jerome-Majewska, L. A. & Papaioannou, V. E. (2004) Hum. Mol. Genet. 13, 2829-2840. [DOI] [PubMed] [Google Scholar]
- 26.Liao, J., Kochilas, L., Nowotschin, S., Arnold, J. S., Aggarwal, V. S., Epstein, J. A., Brown, M. C., Adams, J. & Morrow, B. E. (2004) Hum. Mol. Genet. 13, 1577-1585. [DOI] [PubMed] [Google Scholar]
- 27.Raft, S., Nowotschin, S., Liao, J. & Morrow, B. E. (2004) Development (Cambridge, U.K.) 131, 1801-1812. [DOI] [PubMed] [Google Scholar]
- 28.Xu, H., Morishima, M., Wylie, J. N., Schwartz, R. J., Bruneau, B. G., Lindsay, E. A. & Baldini, A. (2004) Development (Cambridge, U.K.) 131, 3217-3227. [DOI] [PubMed] [Google Scholar]
- 29.Moraes, F., Novoa, A., Jerome-Majewska, L. A., Papaioannou, V. E. & Mallo, M. (2005) Mech. Dev. 122, 199-212. [DOI] [PubMed] [Google Scholar]
- 30.Funke, B., Epstein, J. A., Kochilas, L. K., Lu, M. M., Pandita, R. K., Liao, J., Bauerndistel, R., Schuler, T., Schorle, H., Brown, M. C., et al. (2001) Hum. Mol. Genet. 10, 2549-2556. [DOI] [PubMed] [Google Scholar]
- 31.Lee, M., Chen, K., Shih, J. C. & Hiroi, N. (2004) Genes Brain Behav. 3, 216-227. [DOI] [PubMed] [Google Scholar]
- 32.Rueter, L. E., Ballard, M. E., Gallagher, K. B., Basso, A. M., Curzon, P. & Kohlhaas, K. L. (2004) Psychopharmacology 176, 312-319., [DOI] [PubMed] [Google Scholar]
- 33.Tandon, R., Milner, K. & Jibson, M. D. (1999) J. Clin. Psychiatry 60, Suppl. 8, 21-28. [PubMed] [Google Scholar]
- 34.Baldessarini, R. J., Centorrino, F., Flood, J. G., Volpicelli, S. A., Huston-Lyons, D. & Cohen, B. M. (1993) Neuropsychopharmacology 9, 117-124. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto, T., Hashimoto, K., Yasumatsu, H., Tanaka, H., Fujimura, M., Kuriyama, M., Kimura, K., Takehara, S. & Yamagami, K. (2002) Neuropsychopharmacology. 26, 456-467. [DOI] [PubMed] [Google Scholar]
- 36.Lynch, C. B. (1980) Genetics 96, 757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluyter, F., Bult, A., Lynch, C. B., van Oortmerssen, G. A. & Koolhaas, J. M. (1995) Behav. Genet. 25, 247-252. [DOI] [PubMed] [Google Scholar]
- 38.Miyakawa, T., Leiter, L. M., Gerber, D. J., Gainetdinov, R. R., Sotnikova, T. D., Zeng, H., Caron, M. G. & Tonegawa, S. (2003) Proc. Natl. Acad. Sci. USA 100, 8987-8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meltzer, H. Y. (1995) in Psychopharmacology: The Fourth Generation of Progress, eds. Bloom, F. E. & Kupfer, D. J. (Raven, New York), pp. 1277-1286.
- 40.Gothelf, D., Frisch, A., Munitz, H., Rockah, R., Laufer, N., Mozes, T., Hermesh, H., Weizman, A. & Frydman, M. (1999) Schizophr. Res. 35, 105-112. [DOI] [PubMed] [Google Scholar]
- 41.Usiskin, S. I., Nicolson, R., Krasnewich, D. M., Yan, W., Lenane, M., Wudarsky, M., Hamburger, S. D. & Rapoport, J. L. (1999) J. Am. Acad. Child Adolesc. Psychiatry 38, 1536-1543. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee, J., Christian, B. T., Narayanan, T. K., Shi, B. & Mantil, J. (2001) Neuropsychopharmacology 25, 476-488. [DOI] [PubMed] [Google Scholar]
- 43.Kapur, S., VanderSpek, S. C., Brownlee, B. A. & Nobrega, J. N. (2003) J. Pharmacol. Exp. Ther. 305, 625-631. [DOI] [PubMed] [Google Scholar]
- 44.Gleason, S. D. & Shannon, H. E. (1997) Psychopharmacology 129, 79-84. [DOI] [PubMed] [Google Scholar]
- 45.Mohn, A. R., Gainetdinov, R. R., Caron, M. G. & Koller, B. H. (1999) Cell 98, 427-436. [DOI] [PubMed] [Google Scholar]
- 46.Moore, N. A. (1999) Br. J. Psychiatry 174, Suppl. 38, 5-11. [PubMed] [Google Scholar]
- 47.Xu, R., Hranilovic, D., Fetsko, L. A., Bucan, M. & Wang, Y. (2002) Mol. Psychiatry 7, 1075-1082. [DOI] [PubMed] [Google Scholar]
- 48.Crawley, J. N. (2000) What's Wrong with My Mice? Behavioral Phenotyping of Transgenic and Knockout Mice (Wiley-Liss, New York).
- 49.Ballard, T. M., Pauly-Evers, M., Higgins, G. A., Ouagazzal, A. M., Mutel, V., Borroni, E., Kemp, J. A., Bluethmann, H. & Kew, J. N. (2002) J Neurosci. 22, 6713-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolkman, M. J., Streijger, F., Linkels, M., Bloemen, M., Heeren, D. J., Hendriks, W. J. & Van der Zee, C. E. (2004) Behav. Brain Res. 154, 171-182. [DOI] [PubMed] [Google Scholar]
- 51.Yagi, H., Furutani, Y., Hamada, H., Sasaki, T., Asakawa, S., Minoshima, S., Ichida, F., Joo, K., Kimura, M., Imamura, S., et al. (2003) Lancet 362, 1366-1373. [DOI] [PubMed] [Google Scholar]
- 52.Stoller, J. Z. & Epstein, J. A. (2005) Hum. Mol. Genet. 14, 885-892. [DOI] [PubMed] [Google Scholar]
- 53.Yui, K., Goto, K., Ikemoto, S., Ishiguro, T., Angrist, B., Duncan, G. E., Sheitman, B. B., Lieberman, J. A., Bracha, S. H. & Ali, S. F. (1999) Mol. Psychiatry 4, 512-523. [DOI] [PubMed] [Google Scholar]
- 54.Done, D. J., Crow, T. J., Johnstone, E. C. & Sacker, A. (1994) Brit. Med. J. 309, 699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones, P., Rodgers, B., Murray, R. & Mamot, M. (1994) Lancet 344, 1398-1402. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto, Y., Yamada, K., Noda, Y., Mori, H., Mishina, M. & Nabeshima, T. (2001) J. Neurosci. 21, 750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stefansson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Sigmundsson, T., Ghosh, S., Brynjolfsson, J., Gunnarsdottir, S., Ivarsson, O., Chou, T. T., et al. (2002) Am. J. Hum. Genet. 71, 877-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javitt, D. C. (2004) Mol. Psychiatry 9, 984-997. [DOI] [PubMed] [Google Scholar]
- 59.Vollenweider, F. X. & Geyer, M. A. (2001) Brain Res. Bull. 56, 495-507. [DOI] [PubMed] [Google Scholar]
- 60.Andreasen, N. C. (2000) Brain Res. Rev. 31, 106-112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.