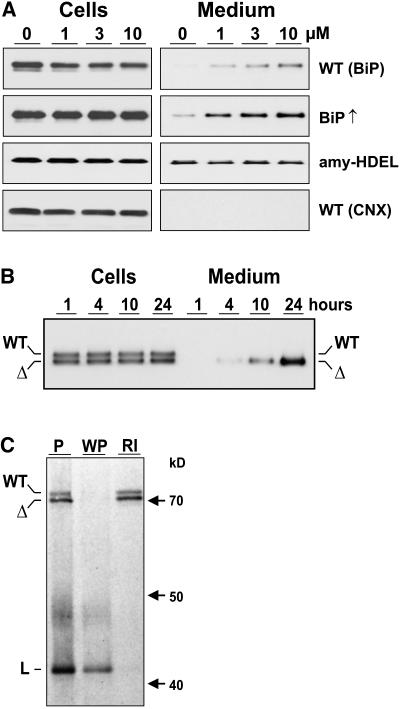
Figure 1.
Wortmannin Induces BiP Secretion.
(A) Immunodetection of BiP in cells and 10-fold concentrated medium from tobacco wild-type protoplasts (WT BiP) incubated with an increasing concentration of wortmannin given in micromoles above the lanes. The second panel shows the result of the same type of experiment but from protoplasts prepared from stable BiP overproducing transgenic plants (BiP↑) (Leborgne-Castel et al., 1999). The third panel shows results from protoplasts prepared from transgenic plants producing recombinant α-amylase-HDEL (amy-HDEL). The bottom panel shows samples from the top panel but probed with anticalnexin antiserum (WT CNX).
(B) Secretion as a function of time in protoplast suspensions prepared from transgenic plants producing BiP (WT) and BiPΔHDEL (Δ) (Leborgne-Castel et al., 1999) incubated with 10 μM wortmannin. Sampling was performed as in (A) except that equal levels of cells and medium were loaded. Numbers above the lanes refer to time of protoplast incubation until harvesting.
(C) Coimmunoprecipitation and ATP-mediated release of BiP and BiPΔHDEL from a model ligand (L). Protoplasts prepared from a transgenic plant producing both wild-type BiP (WT) and truncated BiPΔHDEL (Δ) were transfected with a plasmid encoding a secreted form of green fluorescent protein (GFP) fused to the P-domain of calreticulin (sGFP-P), previously known to be a strong BiP-ligand (Brandizzi et al., 2003). After 5 h of expression, cells were labeled for 2 h and cell extracts immunoprecipitated with anti-GFP serum. One pellet was washed with ATP release buffer, followed by a reimmunoprecipitation of the released material with anti-BiP serum. Shown is the unwashed pellet (P), the washed pellet (WP), and the reimmunoprecipitation from the washing medium (RI). Molecular mass markers are given in kilodaltons. Note that both BiP and BiPΔHDEL bind to the ligand in an ATP-sensitive manner typical for this chaperone.