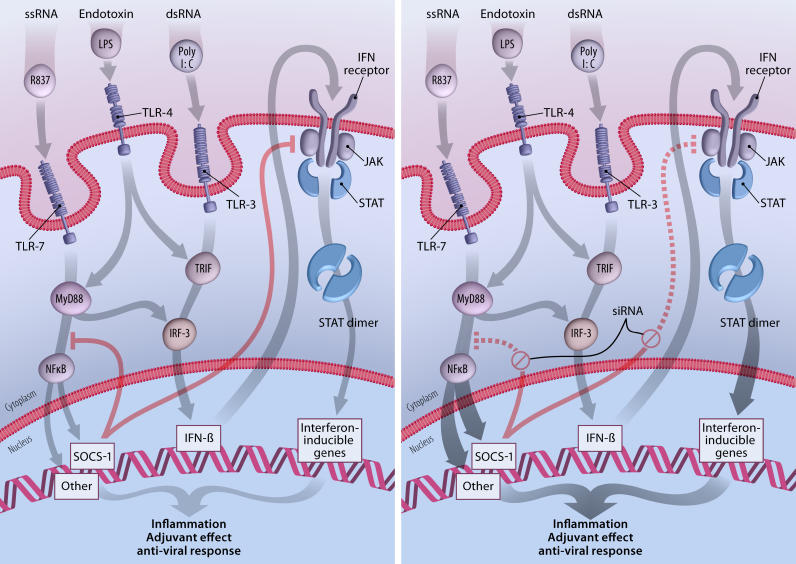
Figure 1. SOCS “Silencing” Enhances the Adjuvant Effect of TLR Ligands.
Substances common in viral pathogens (substances such as single-stranded RNA [ssRNA] and double-stranded RNA [dsRNA]) or in bacteria (substances such as endotoxin) are recognized by TLRs as pathogen-associated molecular patterns. When the TLRs are triggered, a series of signaling events occur that is simplified and schematized in this figure. These events lead to inflammation and activation of innate and adaptive immune responses. SOCS family members are also activated, and serve as an internal control to diminish the intensity and duration of inflammation. In the left-hand panel, R837 mimics ssRNA as a ligand for TLR-7, and initiates signaling through the MyD88 pathway, eventually resulting in the release of NF-κB and in the upregulation of SOCS1 and many genes involved in inflammation. PolyI:C is a synthetic mimic of dsRNA and triggers TLR-3-associated JAK/signal transduction and activator-of-transcription (STAT) signaling through TIR domain-containing adapter-inducing interferon (TRIF), activation of interferon regulatory factor (IRF)-3, and increased production of type 1 interferon. LPS can activate both pathways. SOCS1 specifically interferes with JAK2 and may also inhibit a step between MyD88 and NF-κB release into the nucleus, although this is controversial. This balanced internal feedback mechanism results in a controlled inflammatory process with adjuvant and antiviral effects. When SOCS1 production is blocked by siRNA (right-hand panel), the control of the inflammatory process is temporarily lost, leading to a greater adjuvant and antiviral response that appears to improve vaccine-induced immune responses.
(Illustration: Giovanni Maki)