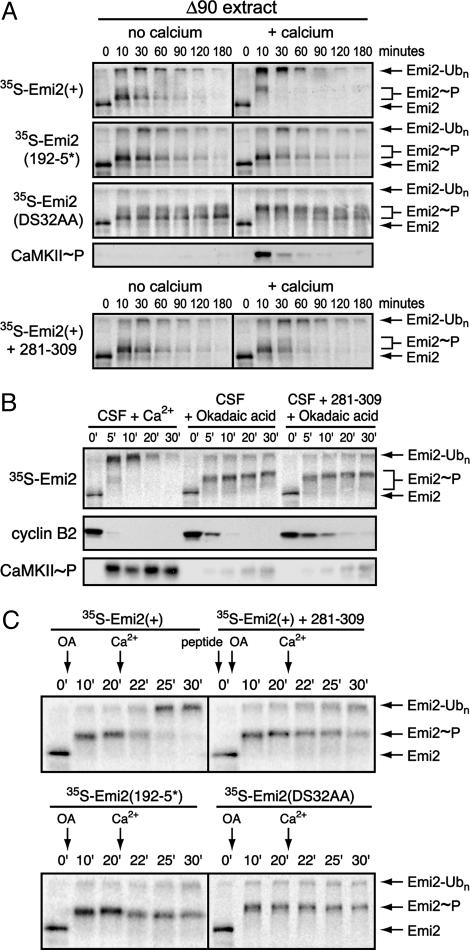
Fig. 4.
Multiple activities contribute to Emi2 stability and function in CSF extract. (A) Emi2 is destroyed in Δ90 mitotic extract in a CaMKII-independent manner, and Ca2+ addition stimulates its rate of destruction. Radiolabeled IVT Emi2 variants were incubated in Δ90 extract with or without Ca2+ addition and with or without 281–309 peptide. Emi2 stability and gel mobility were monitored by autoradiography, and CaMKII activation was detected by immunoblotting. The phospho-CaMKII blot is representative of all ±Ca2+ pairs except those involving the 281–309 peptide, for which no signal for phospho-CaMKII was detected. (B) Phosphatase inhibition in CSF extract causes cyclin B destruction independent of CaMKII activation and Emi2 destruction. CSF extracts were stimulated with either Ca2+ or okadaic acid with or without 281–309 peptide. The stability and gel mobility of radiolabeled IVT Emi2 were monitored by autoradiography. Destruction of cyclin B and activation of CaMKII were monitored by immunoblotting. (C) Emi2 destruction can be induced in okadaic acid-treated CSF extracts, which requires CaMKII and the 192RSST and 32DSGYSDS motifs of Emi2. CSF extracts previously stimulated to destroy cyclin by phosphatase inhibition with or without 281–309 peptide were further stimulated by Ca2+ addition. Stability and gel mobility of radiolabeled IVT Emi2 variants were monitored by autoradiography.