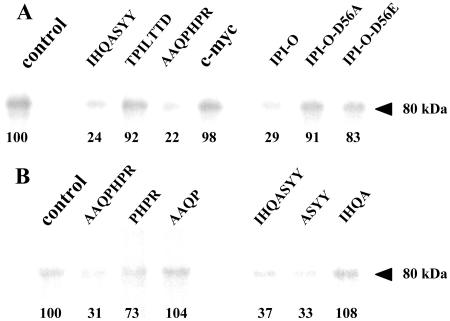
Figure 2.
Photoaffinity labeling of plasma membrane proteins from Arabidopsis with an azido RGD heptapeptide derivative, and effect of the RGD-binding heptapeptides selected by phage display. Plasma membrane proteins were separated in SDS-11% polyacrylamide gels after photolysis of the radioiodinated RGD-photoaffinity probe as described in “Materials and Methods.” Autoradiography revealed label associated with a protein of 80 kD. To quantify cross-linking, gel slices corresponding to the 80-kD polypeptide were excised and γ counted. The values of remaining label are indicated below each band as percentages of the control that was set at 100% in each independent experiment. Fifty micrograms of protein were deposited in each lane. A, Competition by isolated RGD-binding heptapeptides (IHQASYY, TPILTTD, AAQPHPR), a c-myc peptide with unrelated sequence (EQKLISEEDL; each peptide was applied at the concentration of 100 μm), and wild-type and mutated (-D56A, -D56E) MBP-IPI-O proteins (each protein was applied at the concentration of 0.3 μm). B, Competition by the two active RGD-binding heptapeptides (AAQPHPR and IHQASYY) and derived tetrapeptides. Each peptide was applied at the concentration of 100 μm.