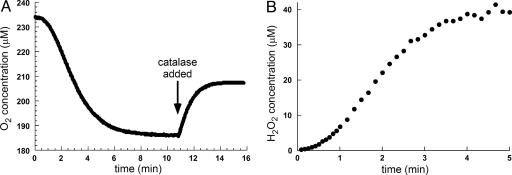
Fig. 4.
Oxygen is reduced by Ero1p activity to form hydrogen peroxide. (A) The reaction was initiated at time 0 by injecting the shorter construct of Ero1p to a final concentration of 1 μM into a solution containing 50 μM Trxred, and oxygen consumption was monitored. Approximately half of the oxygen originally consumed during Trxred oxidation was restored with the addition of catalase (arrow), indicating that oxygen has been reduced to H2O2 in the initial reaction. Similar results were obtained for the longer construct. (B) The reaction was initiated at time 0 by injecting the shorter form of Ero1p to a final concentration of 1 μM into a rapidly stirred solution containing 100 μM Trxred. Samples were withdrawn and mixed with the PeroXOquant detection reagent. Absorbance values were converted to H2O2 concentration according to a standard H2O2 calibration curve.