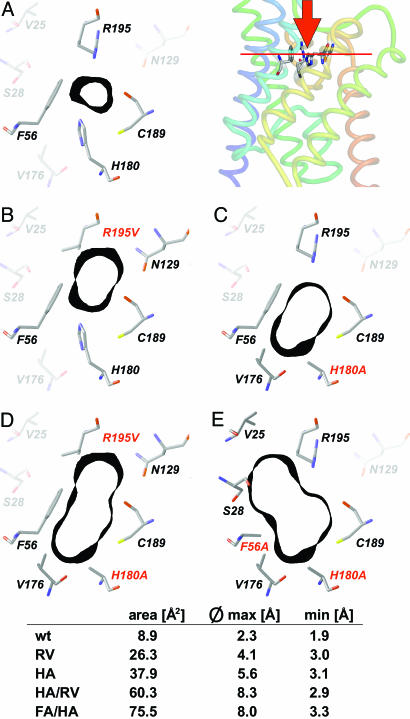
Fig. 1.
Shape of the ar/R constriction of AQP1 and mutants. For orientation, Top Right depicts a side view of the crystal structure of AQP1 (1J4N) with F56, H180, C189, and R195 as sticks (numbering according to the rat sequence; pymol software, DeLano Scientific, San Francisco). The red bar indicates the plane shown in the cross sections A–E.(A) Wild-type ar/R constriction. Amino acid residues that directly contribute to the constriction are drawn in full color. The black bands denote 1-Å-thick sections of the Connolly surface at this site (sybyl software, Tripos Associates, St. Louis). (B–E) Mutant outlines of ar/R constriction with individual mutations printed red. Calculations were based on the bovine AQP1 crystal structure (1J4N) and energy-optimized by using the sybyl threading algorithms. For additional description, see text.