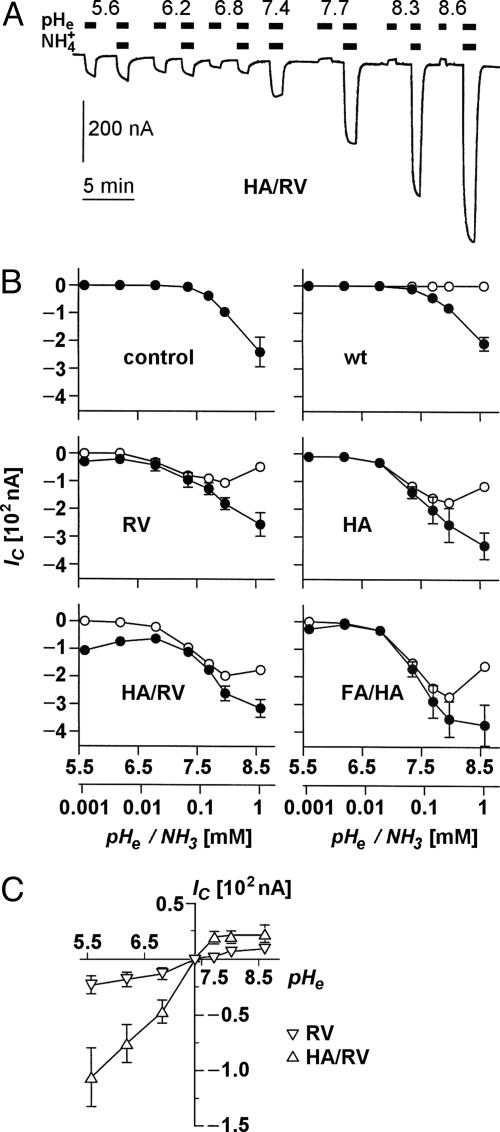
Fig. 4.
Ammonia- and pH-induced proton currents in Xenopus oocytes expressing AQP1 wild-type and mutants. (A) Sample trace of clamp current (-50 mV) induced in an AQP1-H180A/R195V-expressing oocyte by changes in pHe and 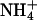 of the bathing solution. The baseline current was -100 nA. pHe was intermittently changed from 7.4 (control) to a range of values from 5.6 to 8.6 ± a concurrent change in
of the bathing solution. The baseline current was -100 nA. pHe was intermittently changed from 7.4 (control) to a range of values from 5.6 to 8.6 ± a concurrent change in 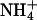 (5 mM Na+ was replaced by 5 mM
(5 mM Na+ was replaced by 5 mM 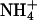 ), as indicated by the bars on top. In the acidic range, pHe changes resulted in inward currents that were independent of the presence of
), as indicated by the bars on top. In the acidic range, pHe changes resulted in inward currents that were independent of the presence of 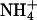 . In the alkaline range, changes in pHe resulted in small outward currents, whereas simultaneous addition of
. In the alkaline range, changes in pHe resulted in small outward currents, whereas simultaneous addition of 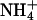 induced large inward currents. The LP of the oocyte was 9.6·10-3 cm·s-1. (B) Filled symbols: total clamp currents (-50 mV) induced by 5 mM
induced large inward currents. The LP of the oocyte was 9.6·10-3 cm·s-1. (B) Filled symbols: total clamp currents (-50 mV) induced by 5 mM 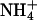 at various pHe values in uninjected oocytes (control) and oocytes expressing AQP1 wild-type and mutants. The open symbols represent the currents induced by AQP-facilitated ammonia passage, i.e., we subtracted the background current induced by ammonia diffusion (control, Top Left) and the current produced by ammonia-independent proton permeation of certain AQP1 mutants (depicted in C). (C) Clamp currents elicited exclusively by changes in pHe. For clarity, we show only the currents for AQP1-R195V and AQP1-H180A/R195V. The currents for AQP1 wild-type and the AQP1-H180A mutant were not significantly different from the miniscule currents already observed in uninjected control oocytes. The current for AQP1-F56A/H180A (not shown) was only significantly different at pHe 5.6, i.e., 17 ± 2 nA (n > 5 for AQPs and n = 3 for uninjected controls).
at various pHe values in uninjected oocytes (control) and oocytes expressing AQP1 wild-type and mutants. The open symbols represent the currents induced by AQP-facilitated ammonia passage, i.e., we subtracted the background current induced by ammonia diffusion (control, Top Left) and the current produced by ammonia-independent proton permeation of certain AQP1 mutants (depicted in C). (C) Clamp currents elicited exclusively by changes in pHe. For clarity, we show only the currents for AQP1-R195V and AQP1-H180A/R195V. The currents for AQP1 wild-type and the AQP1-H180A mutant were not significantly different from the miniscule currents already observed in uninjected control oocytes. The current for AQP1-F56A/H180A (not shown) was only significantly different at pHe 5.6, i.e., 17 ± 2 nA (n > 5 for AQPs and n = 3 for uninjected controls).