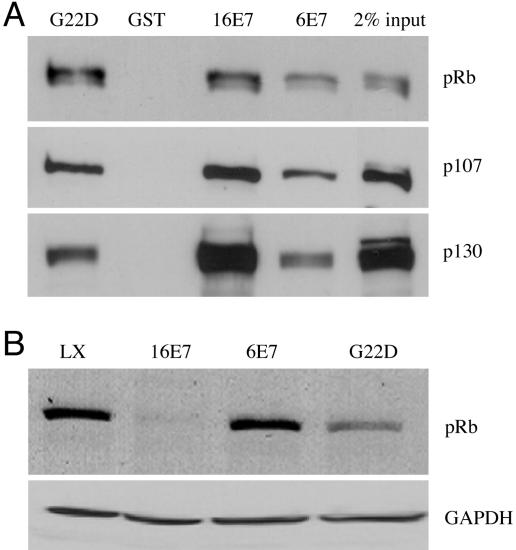
Fig. 1.
Characterization of HPV-6 E7G22D with respect to binding of pRB family members and degradation of pRB. (A) The ability of HPV-6, HPV-16, and HPV-6 E7G22D to bind pRB family members. Jurkat nuclear extracts were incubated with GST, GST-16E7, GST-6E7, or GST-6E7G22D, and bound proteins were detected after separation by SDS/PAGE and transfer to nitrocellulose membrane. The membrane was probed with antibodies to pRB, p107, and p130. Ponceau red staining of the membrane before probing indicated that similar levels of GST or GST fusion proteins were present in the precipitate (data not shown). The average ± SD of bound pRB in three independent experiments, corrected for Ponceau red and relative to GST16E7 (set to 1.0), was 1.22 ± 0.15 (G22D) and 0.35 ± 0.06 (6E7); the average ± SD of bound p107 was 0.73 ± 0.08 (G22D) and 0.36 ± 0.01 (6E7); the average of bound p130 was 0.42 ± 0.07 (G22D) and 0.23 ± 0.05 (6E7). (B) Immunoblot of pRB in the presence and absence of E7. HFKs were infected with control retrovirus LXSN, L(16E7)SN, or L(6E7)SN and grown in C-K-SFM. Whole-cell lysates were analyzed by immunoblot by using antibodies to pRB and GAPDH, the latter as a loading control. The average ± SD of pRB in three independent experiments, corrected for GAPDH and relative to LXSN, was 0.24 ± 0.14 (16E7), 0.89 ± 0.32 (6E7), and 0.50 ± 0.19 (G22D).