Figure 3.
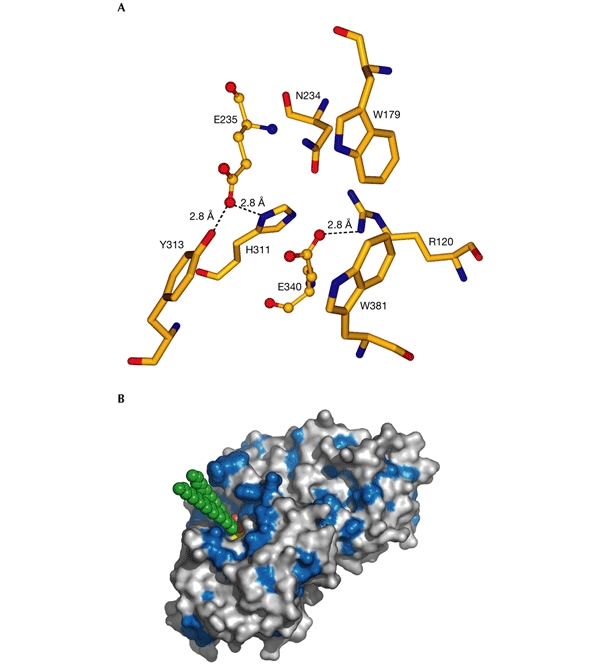
Active site of acid-β-glucosidase. (A) The catalytic and glucone-binding site of acid-β-glucosidase (GlcCerase). The catalytic glutamates are shown as ball-and-stick models and amino-acid residues nearby are shown as sticks. Hydrogen bonds are shown as dashed lines for those residues close enough to contact the glutamates. These residues may be involved directly in catalysis or may modulate the protonation states of the carboxyl groups. The others residues are near the docked glucosyl moiety (see (B)), and these may thus stabilize its interaction with GlcCerase. (B) Three-dimensional surface diagram of GlcCerase (created using PyMOL (http://www.pymol.org)), with a model of the docked substrate (based on the coordinates of galactosylceramide (Nyholm et al., 1990) and modified for GlcCer). Hydrophobic residues (W, F, Y, L, I, V, M and C; Hopp & Woods, 1981) are shown in blue, and the activesite residues (E235 and E340) in yellow. GlcCer is shown in CPK format (carbon atoms in green, and oxygen atoms in red).
