Figure 4.
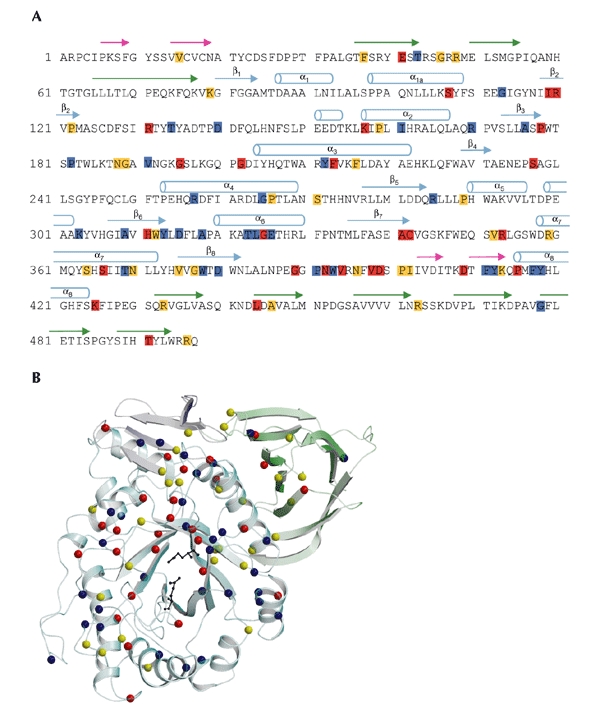
Mutations in acid-β-glucosidase. (A) The sequence of the 497 residues of acid-β-glucosidase (GlcCerase). Mutations reported to cause severe disease (http://www.tau.ac.il/~racheli/genedis/gaucher/gaucher.html) are shown in red, those that cause mild disease in yellow, and those for which clinical data documenting severity of the disease are lacking in blue. Only single amino-acid substitutions are included, with frameshifts and splices excluded as the enzyme is not expressed in most of these cases. Helices are indicated by cylinders, and β-strands are indicated by arrows of colours corresponding to those of the domains shown in Fig. 2. (B) Distribution in the three-dimensional structure of GlcCerase of single amino-acid substitutions that lead to Gaucher disease. Colour coding is the same as in (A). In some cases, assignment of phenotypes as mild (type 1) or severe (types 2 and 3) is based on a few individuals, and sometimes only on one. The phenotypes of several mutations are not known, as the mutations were detected in genomic DNA, and data about disease severity may not have been available. The activesite glutamate residues are shown as black sticks. Cerezyme® differs from GlcCerase by a single amino-acid substitution at residue 495 (His for Arg).
