Abstract
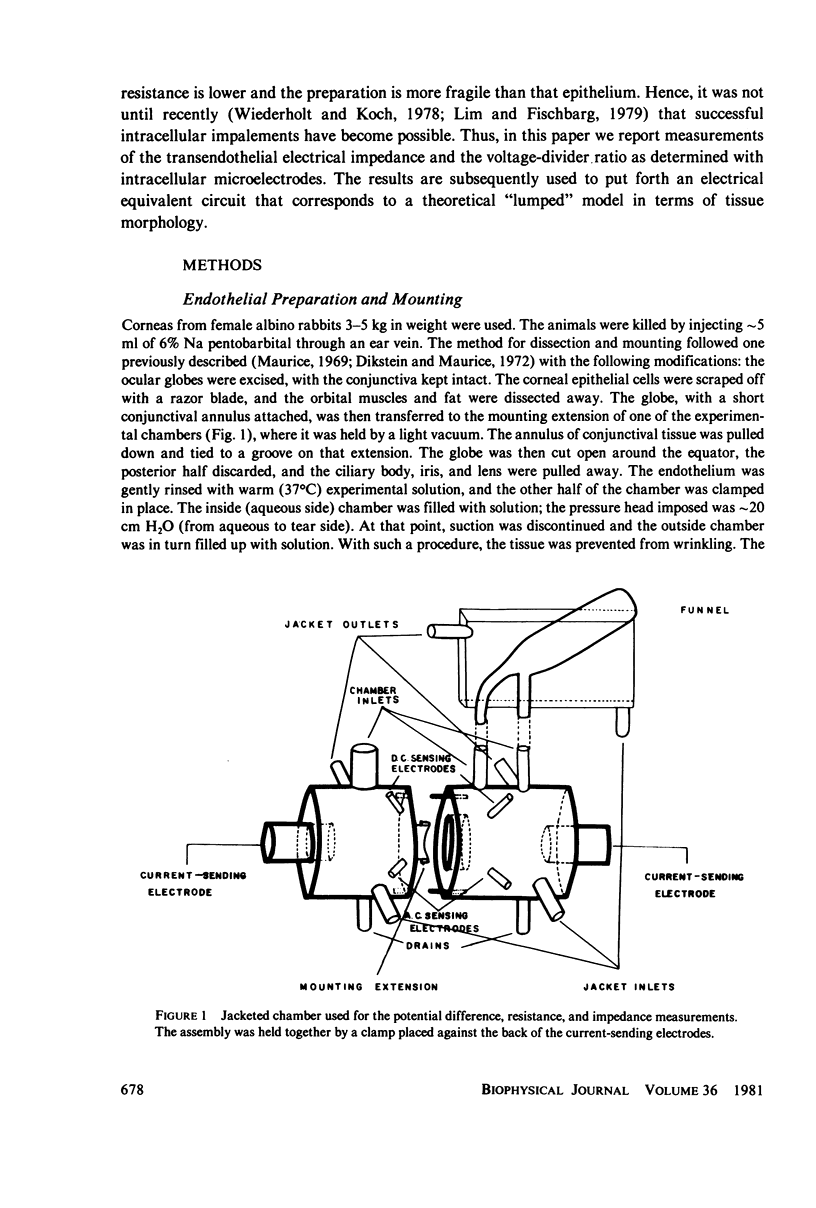
Alternating- and direct-current electrical characteristics of rabbit corneal endothelium were studied under varying experimental conditions. The measurements were performed by sending a 10-microA current (AC or DC) across the tissue layer. Maximal values of transendothelial potential difference and resistance were 1.3 +/- 0.1 mV and 73 +/- 6 omega . cm2, respectively. The short-circuit current was estimated from the potential and resistance values. Impedance loci were obtained for the frequency range 0.5-100 kHz. A capacitive reactance (C = 0.63 +/- 0.02 microF/cm2) was observed in the 100 Hz-100 kHz range. To relate the impedance data to the electrical parameters of the cell membranes, the voltage-divider ratio was determined by sending square pulse across the tissue and measuring voltage responses across the apical and basal membranes with an intracellular microelectrode. The intracellular potential difference was on the average -61 +/- 1 mV, and the voltage-divider ratio was found to be between 0.33 and 4. Impedance data were fit by a computer to an equivalent circuit representing a "lumped" model, and the agreement between the model and the data was satisfactory. The results are discussed in terms of both the morphological characteristics and properties of the fluid transport mechanism across the preparation.
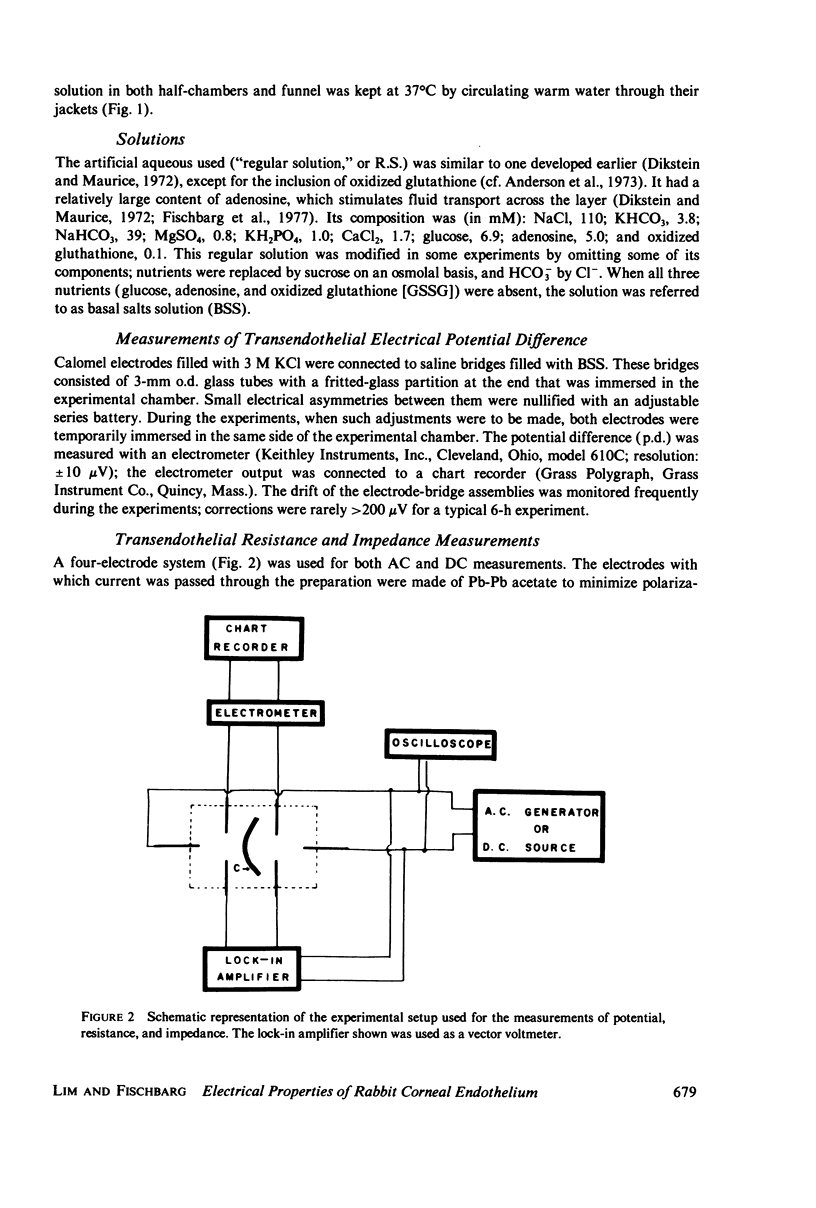
Full text
PDF

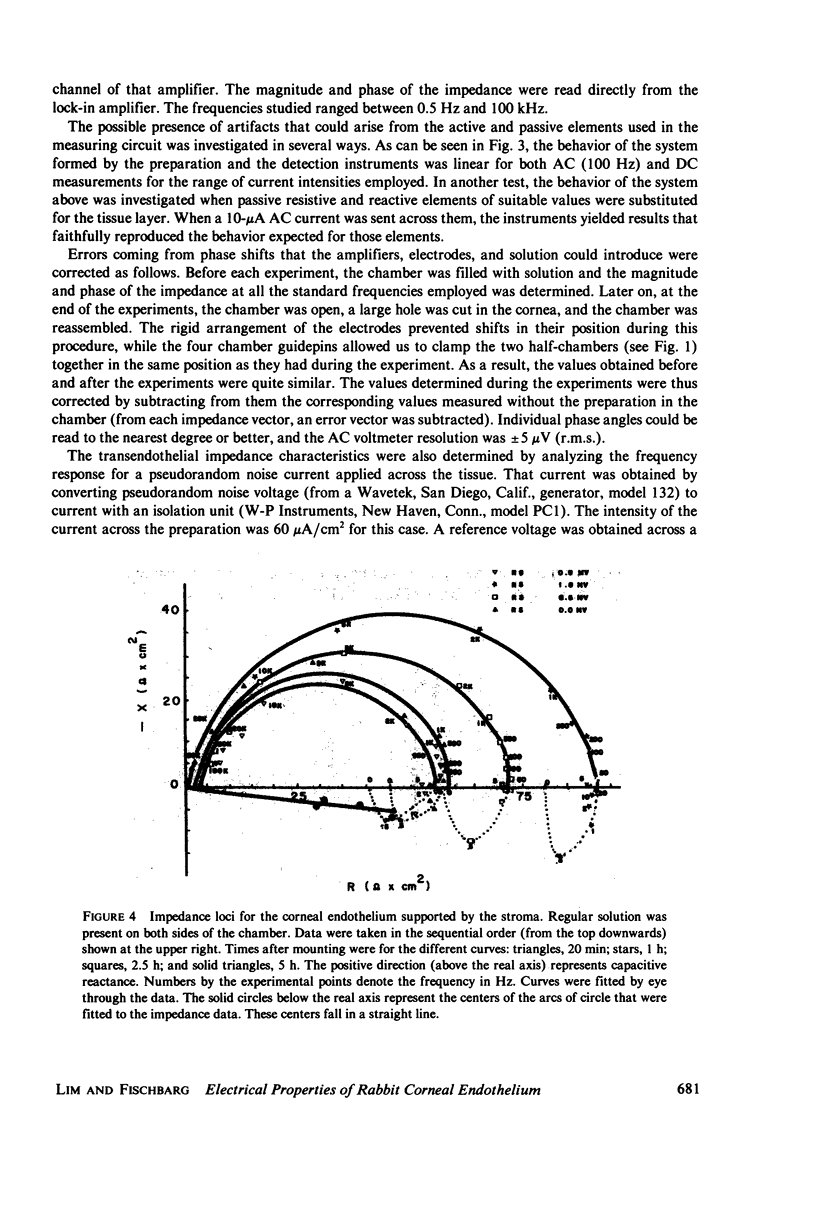










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. I., Fischbarg J., Spector A. Fluid transport, ATP level and ATPase activities in isolated rabbit corneal endothelium. Biochim Biophys Acta. 1973 May 25;307(3):557–562. doi: 10.1016/0005-2736(73)90300-3. [DOI] [PubMed] [Google Scholar]
- Barfort P., Maurice D. Electrical potential and fluid transport across the corneal endothelium. Exp Eye Res. 1974 Jul;19(1):11–19. doi: 10.1016/0014-4835(74)90067-0. [DOI] [PubMed] [Google Scholar]
- Brown A. C., Kastella K. G. The AC impedance of frog skin and its relation to active transport. Biophys J. 1965 Jul;5(4):591–606. doi: 10.1016/S0006-3495(65)86736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
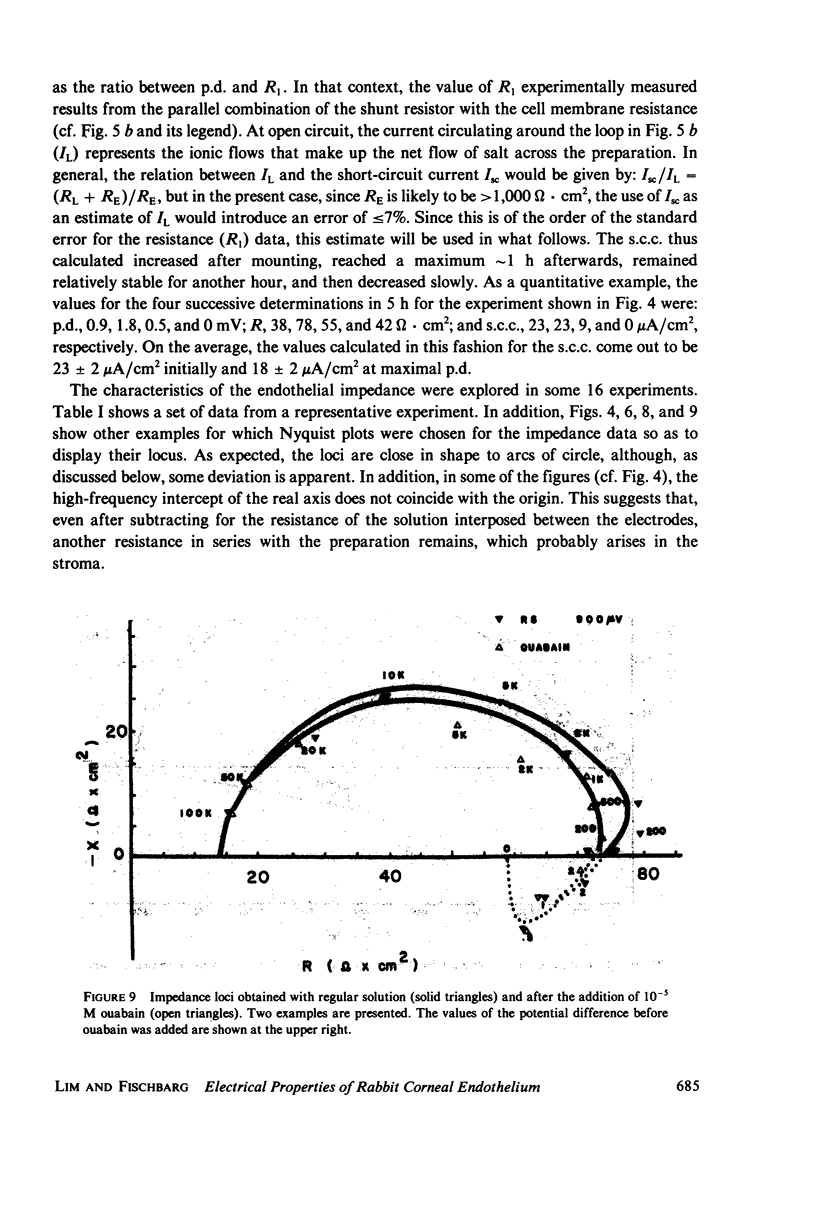
- COLE K. S. ELECTRODIFFUSION MODELS FOR THE MEMBRANE OF SQUID GIANT AXON. Physiol Rev. 1965 Apr;45:340–379. doi: 10.1152/physrev.1965.45.2.340. [DOI] [PubMed] [Google Scholar]
- COLE K. S. Some physical aspects of bioelectric phenomena. Proc Natl Acad Sci U S A. 1949 Oct;35(10):558–566. doi: 10.1073/pnas.35.10.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C., Lewis S. A., Diamond J. M. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979 May;26(2):291–317. doi: 10.1016/S0006-3495(79)85250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Functional consequences of ultrastructural geometry in "backwards" fluid-transporting epithelia. J Cell Biol. 1968 Jun;37(3):694–702. doi: 10.1083/jcb.37.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein S., Maurice D. M. The metabolic basis to the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):29–41. doi: 10.1113/jphysiol.1972.sp009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. Active and passive properties of the rabbit corneal endothelium. Exp Eye Res. 1973 May 10;15(5):615–638. doi: 10.1016/0014-4835(73)90071-7. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J., Bourguet J. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J Membr Biol. 1977 Jun 30;35(2):95–112. doi: 10.1007/BF01869942. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J. Determination of the impedance locus of rabbit corneal endothelium. Biophys J. 1973 Jun;13(6):595–599. doi: 10.1016/S0006-3495(73)86009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J. Role of cations, anions and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol. 1974 Sep;241(3):647–675. doi: 10.1113/jphysiol.1974.sp010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. Potential difference and fluid transport across rabbit corneal endothelium. Biochim Biophys Acta. 1972 Nov 2;288(2):362–366. doi: 10.1016/0005-2736(72)90257-x. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Warshavsky C. R., Lim J. J. Pathways for hydraulically and osmotically-induced water flows across epithelia. Nature. 1977 Mar 3;266(5597):71–74. doi: 10.1038/266071a0. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Van Driessche W. Capacitive and inductive low frequency impedances of Necturus gallbladder epithelium. Pflugers Arch. 1981 Jan;389(2):105–113. doi: 10.1007/BF00582099. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Miller D. A. In vitro techniques for avoiding edge damage in studies of frog skin. Science. 1971 Jul 9;173(3992):146–148. doi: 10.1126/science.173.3992.146. [DOI] [PubMed] [Google Scholar]
- Hirsch M., Renard G., Faure J. P., Pouliquen Y. Formation of intercellular spaces and junctions in regenerating rabbit corneal endothelium. Exp Eye Res. 1976 Oct;23(4):385–397. doi: 10.1016/0014-4835(76)90166-4. [DOI] [PubMed] [Google Scholar]
- Hodson S., Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol. 1976 Dec;263(3):563–577. doi: 10.1113/jphysiol.1976.sp011645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S. The regulation of corneal hydration by a salt pump requiring the presence of sodium and bicarbonate ions. J Physiol. 1974 Jan;236(2):271–302. doi: 10.1113/jphysiol.1974.sp010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull D. S., Green K., Boyd M., Wynn H. R. Corneal endothelium bicarbonate transport and the effect of carbonic anhydrase inhibitors on endothelial permeability and fluxes and corneal thickness. Invest Ophthalmol Vis Sci. 1977 Oct;16(10):883–892. [PubMed] [Google Scholar]
- KAYE G. I., PAPPAS G. D. Studies on the cornea. I. The fine structure of the rabbit cornea and the uptake and transport of colloidal particles by the cornea in vivo. J Cell Biol. 1962 Mar;12:457–479. doi: 10.1083/jcb.12.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyce S. D., Wong R. K. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977 Apr;266(3):777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. J., Fischbarg J. Intra-cellular potential of rabbit corneal endothelial cells. Exp Eye Res. 1979 Jun;28(6):619–626. doi: 10.1016/0014-4835(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Lim J. J. Na+ transport across the rabbit corneal endothelium. Curr Eye Res. 1981;1(4):255–258. doi: 10.3109/02713688109001856. [DOI] [PubMed] [Google Scholar]
- MAURICE D. M. The permeability to sodium ions of the living rabbit's cornea. J Physiol. 1951 Feb;112(3-4):367–391. doi: 10.1113/jphysiol.1951.sp004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D. M. The location of the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAN H. P. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Sandblom J. Anomalous reactances in electrodiffusion systems. Biophys J. 1972 Sep;12(9):1118–1131. doi: 10.1016/S0006-3495(72)86149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferdecker E., Frömter E. The AC impedance of Necturus gallbladder epithelium. Pflugers Arch. 1978 Nov 14;377(2):125–133. doi: 10.1007/BF00582842. [DOI] [PubMed] [Google Scholar]
- Silver G. A., Strauss J., Misrahy G. A. Electrical impedance of isolated amnion. Biophys J. 1965 Nov;5(6):855–865. doi: 10.1016/S0006-3495(65)86756-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. G. The low-frequency electrical impedance of the isolated frog skin. Acta Physiol Scand. 1971 Mar;81(3):355–366. doi: 10.1111/j.1748-1716.1971.tb04910.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Rohlicek V., Frömter E. A quasi-totally shielded, low-capacitance glass-microelectrode with suitable amplifiers for high-frequency intracellular potential and impedance measurements. Pflugers Arch. 1978 Dec 28;378(2):141–148. doi: 10.1007/BF00584447. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Koch M. Intracellular potentials of isolated rabbit and human corneal endothelium. Exp Eye Res. 1978 Nov;27(5):511–518. doi: 10.1016/0014-4835(78)90136-7. [DOI] [PubMed] [Google Scholar]



