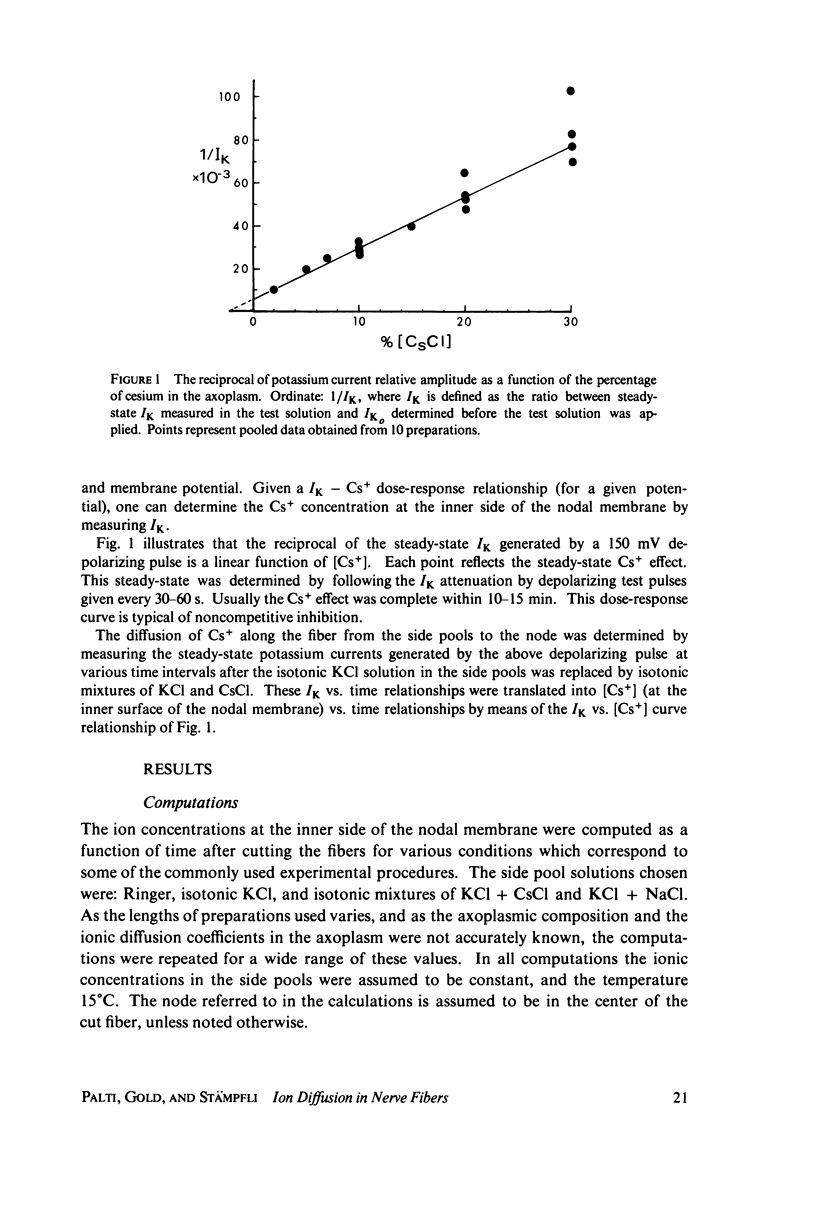
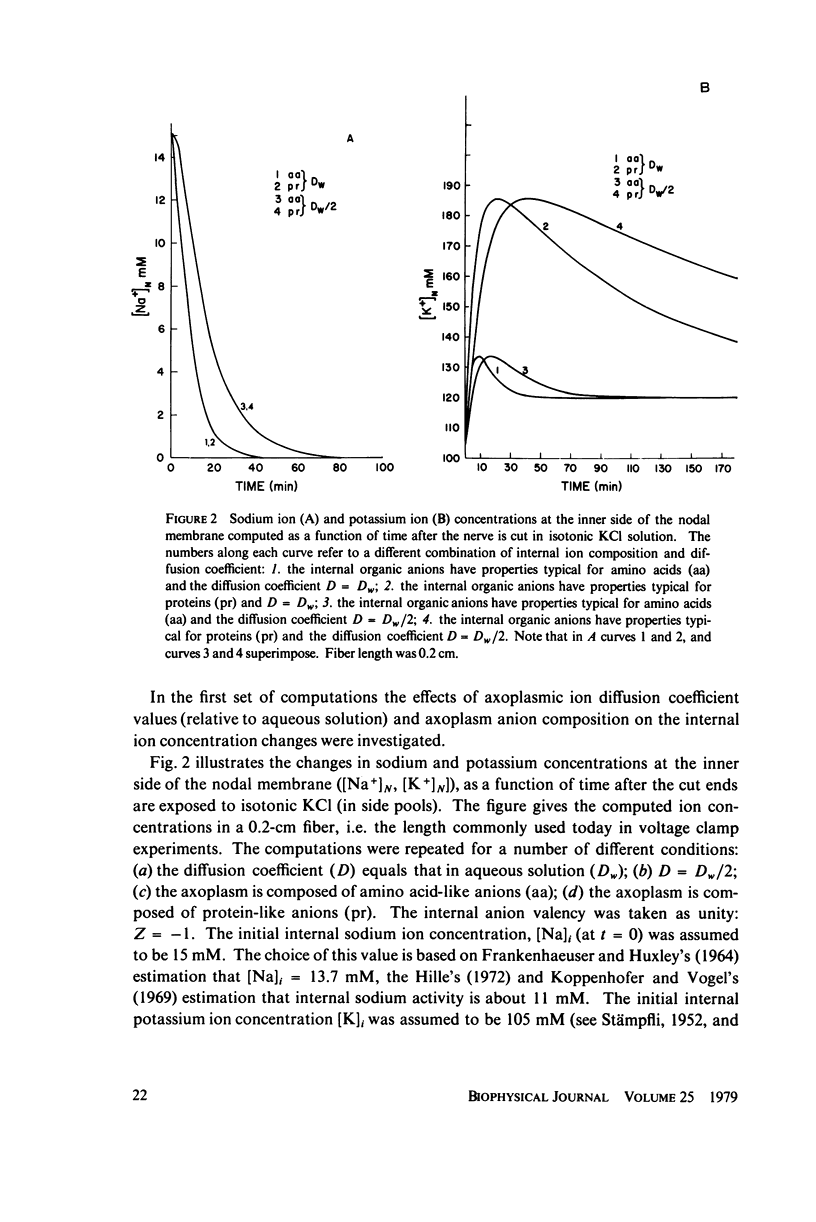
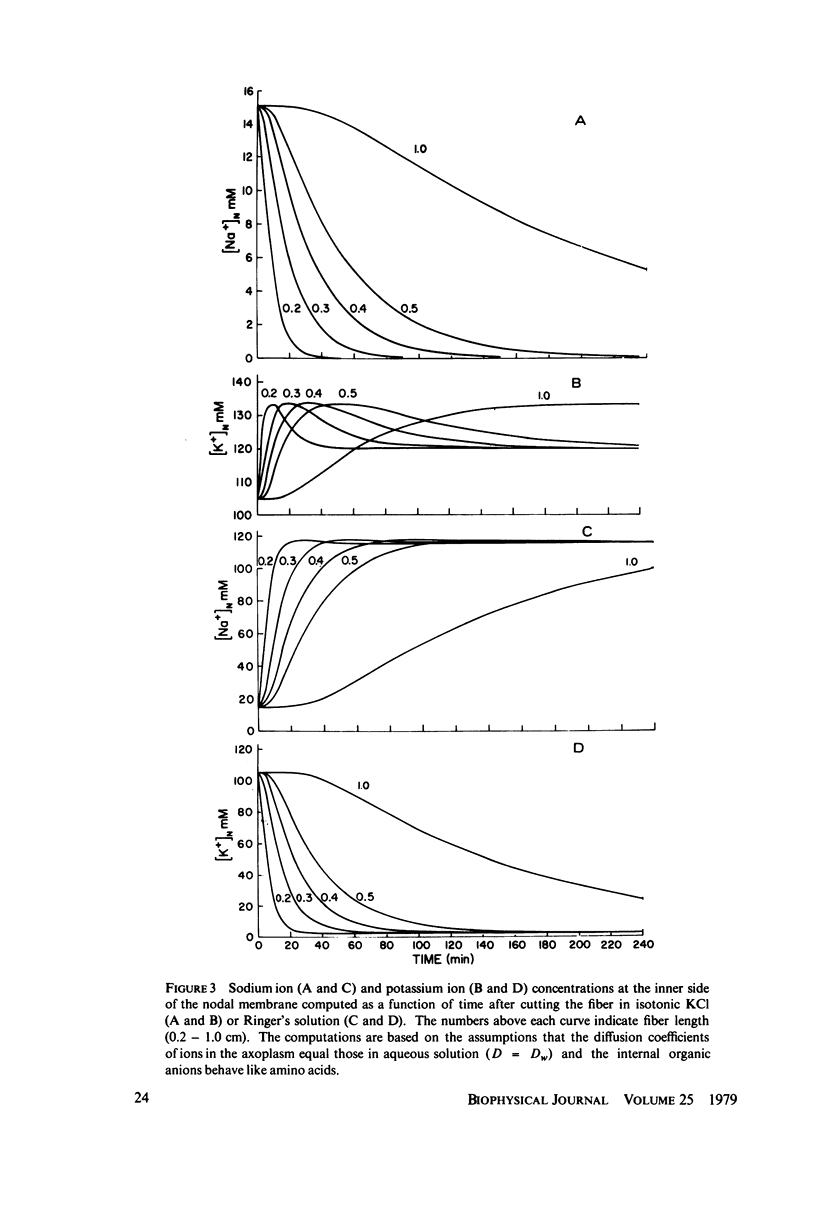
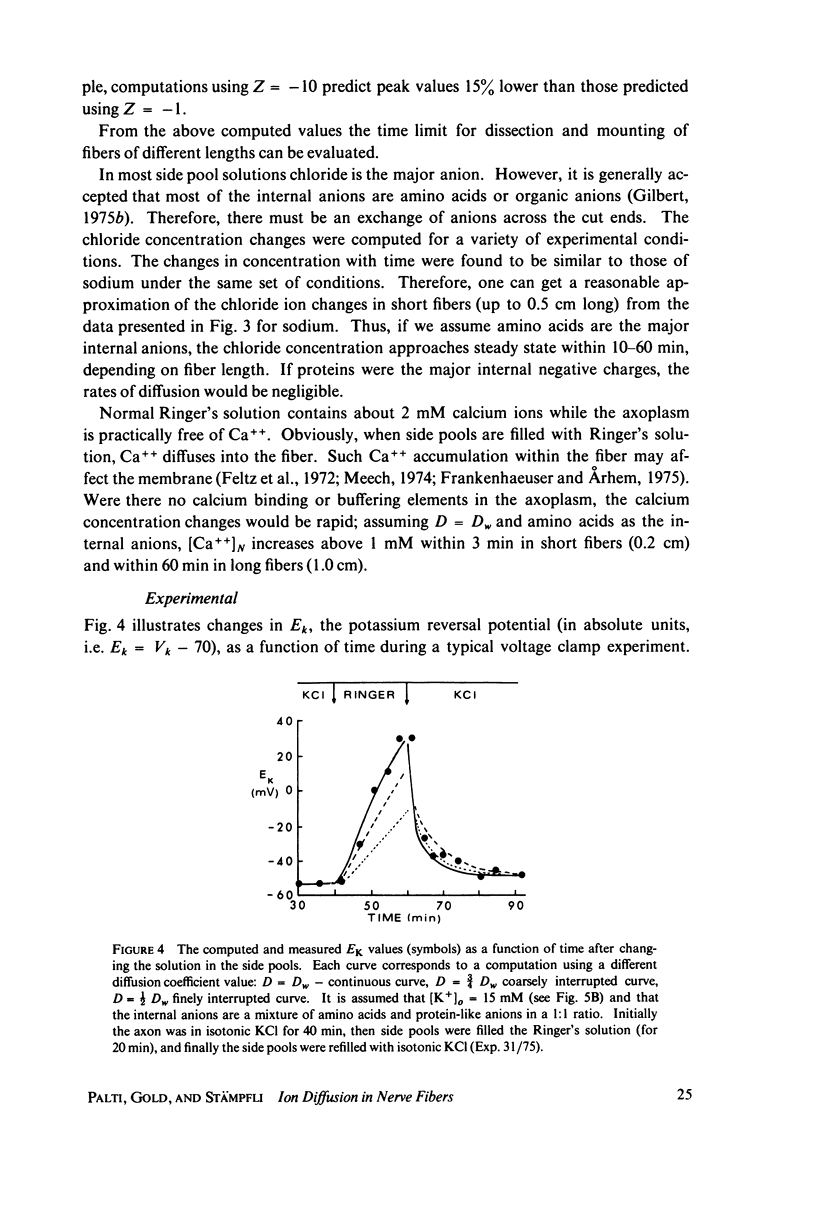
Abstract
The diffusion of ions towards or away from the inner side of the nodal membrane in preparations, the cut ends of which are placed in various media, was investigated. The ion concentration changes were calculated by numerical solution of the unidimensional electrodiffusion equation under a variety of media compositions, axoplasmic diffusion coefficients, and internal anionic compositions. The potassium and cesium ion diffusion along the axon towards the node was determined experimentally by two different electrophysiological methods. On the basis of comparison between the experimental data and the computational predictions the axoplasmic potassium ion diffusion coefficient was determined to be almost equal to that in free aqueous solution, while that of cesium ion was close to one half of that in aqueous solution. Utilizing the values of diffusion parameters thus determined, we solved the electrodiffusion equation for a number of common experimental procedures. We found that in short fibers, cut 0.1-0.2 cm at each side of the node, the concentration approached values close to the new steady-state values within 5-30 min. In long fibers (over 1 cm long) steady-state concentrations were obtained only after a few hours. Under some conditions the internal concentrations transiently overshot the steady-state values. The diffusion potentials generated in the system were also evaluated. The ion concentration changes and generation of diffusion potential cannot be prevented by using side pools with cation content identical to that of the axoplasm.
Full text
PDF




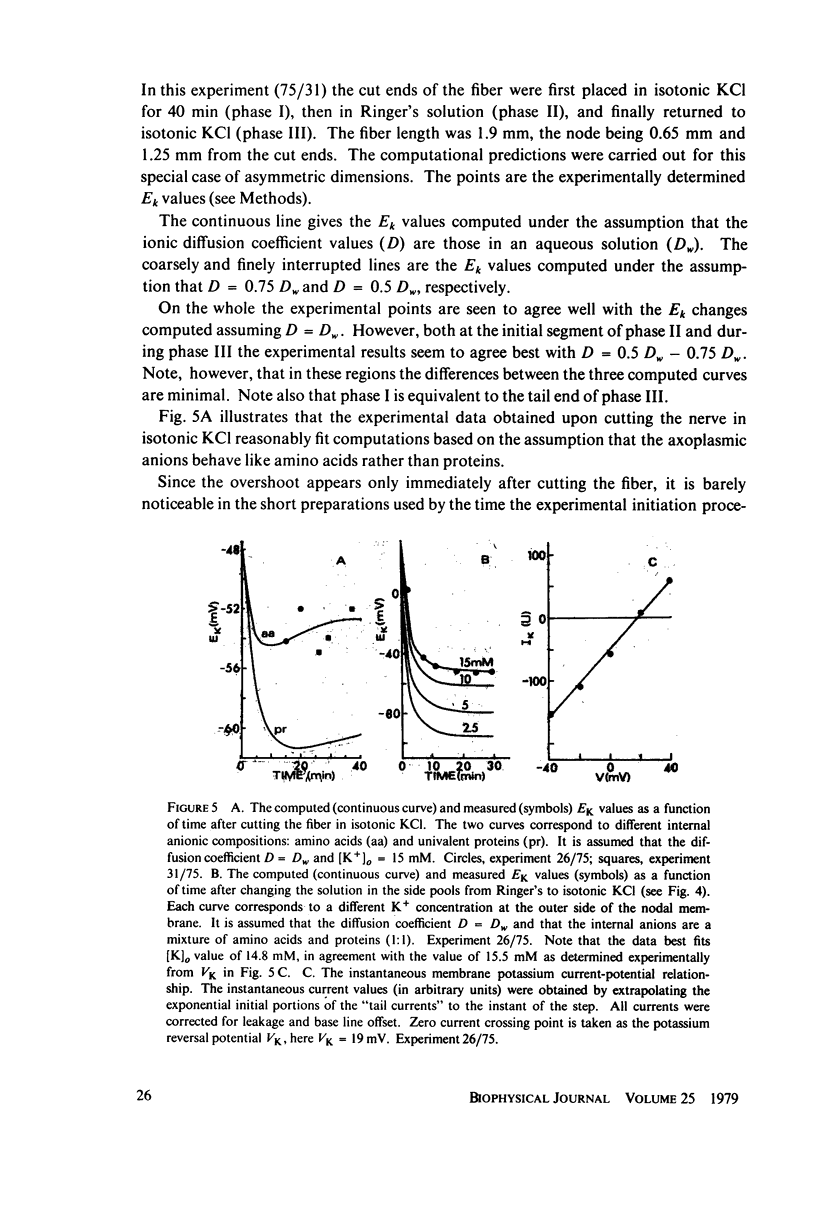




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Arhem P. Diffusion of sodium in axoplasm of myelinated nerve fibre. Potential clamp analysis. Acta Physiol Scand. 1976 Aug;97(4):415–425. doi: 10.1111/j.1748-1716.1976.tb10282.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Hille B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol. 1972 Apr;59(4):388–400. doi: 10.1085/jgp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Cesium induced rectifications in frog myelinated fibres. Pflugers Arch. 1975 Apr 2;355(4):361–364. doi: 10.1007/BF00579857. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Potassium accumulation in the perinodal space of frog myelinated axons. Pflugers Arch. 1975 Jul 21;358(2):111–124. doi: 10.1007/BF00583922. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A method for recording resting and action potentials in the isolated myelinated nerve fibre of the frog. J Physiol. 1957 Mar 11;135(3):550–559. doi: 10.1113/jphysiol.1957.sp005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
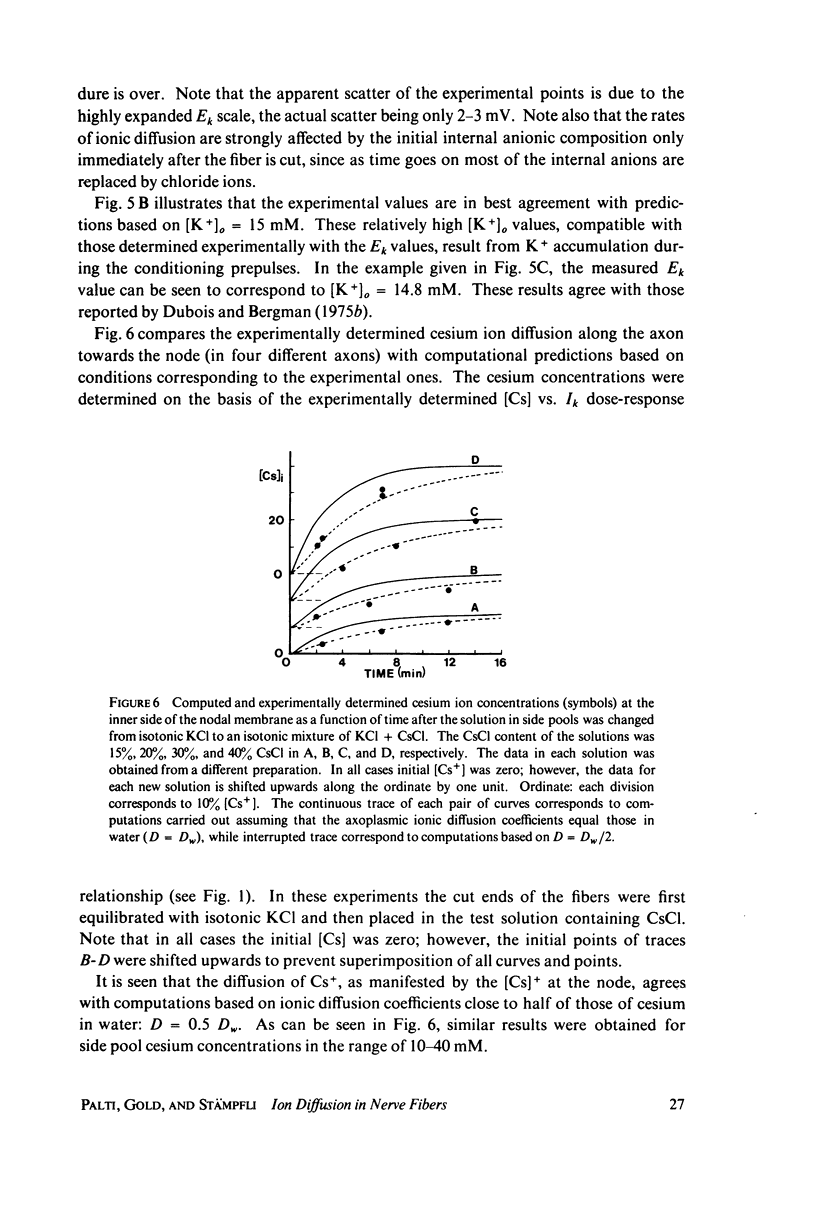
- FRANKENHAEUSER B., HUXLEY A. F. THE ACTION POTENTIAL IN THE MYELINATED NERVE FIBER OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. J Physiol. 1964 Jun;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felz A., Krnjević K., Lisiewicz A. Intracellular free Ca 2+ and membrane properties of motoneurones. Nat New Biol. 1972 Jun 7;237(75):179–181. doi: 10.1038/newbio237179a0. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B., Arhem P. Steady state current rectification in potential clamped nodes of Ranvier (Xenopus laevis). Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):515–525. doi: 10.1098/rstb.1975.0028. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S. Axoplasm architecture and physical properties as seen in the Myxicola giant axon. J Physiol. 1975 Dec;253(1):257–301. doi: 10.1113/jphysiol.1975.sp011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. S. Axoplasm chemical composition in Myxicola and solubility properties of its structural proteins. J Physiol. 1975 Dec;253(1):303–319. doi: 10.1113/jphysiol.1975.sp011191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The mobility and diffusion coefficient of potassium in giant axons from Sepia. J Physiol. 1953 Mar;119(4):513–528. doi: 10.1113/jphysiol.1953.sp004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol. 1972 Jun;59(6):637–658. doi: 10.1085/jgp.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli H. Displacement currents in the node of Ranvier. Voltage and time dependence. Pflugers Arch. 1975;354(1):1–18. doi: 10.1007/BF00584499. [DOI] [PubMed] [Google Scholar]


