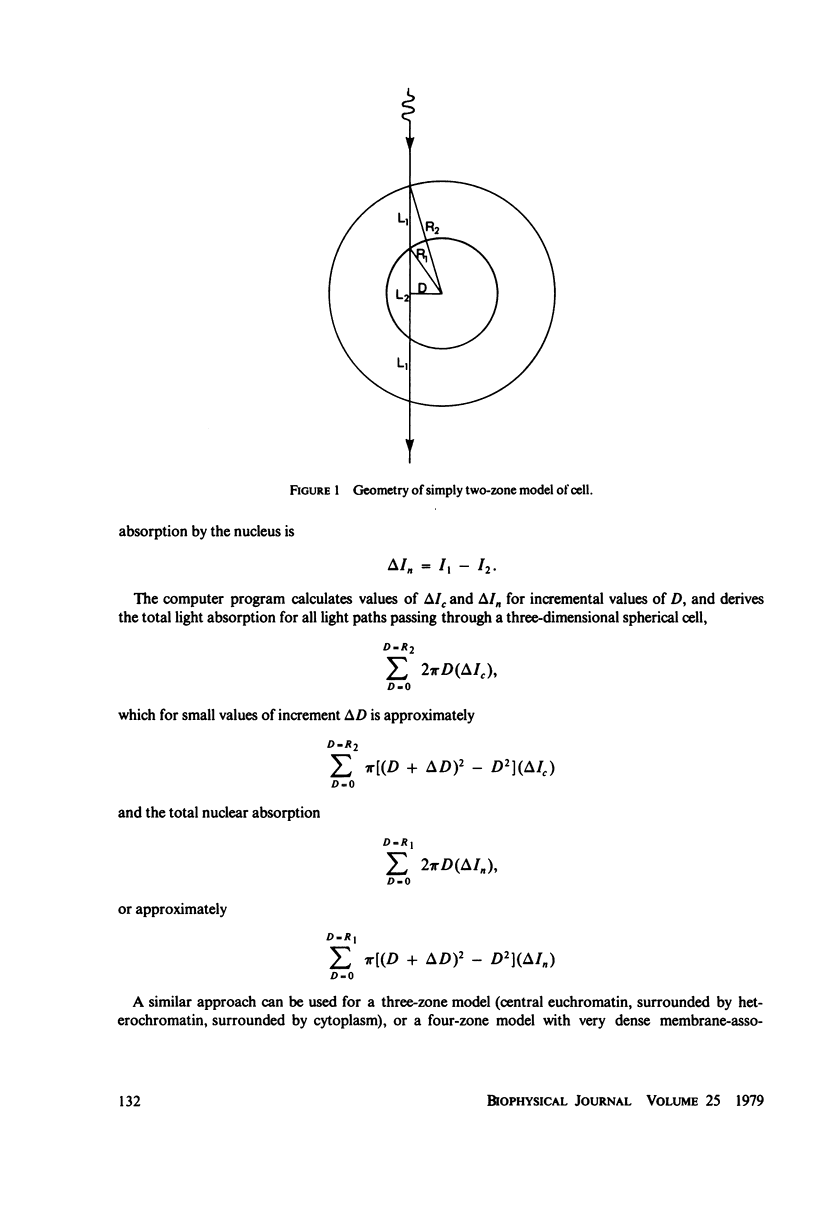
Abstract
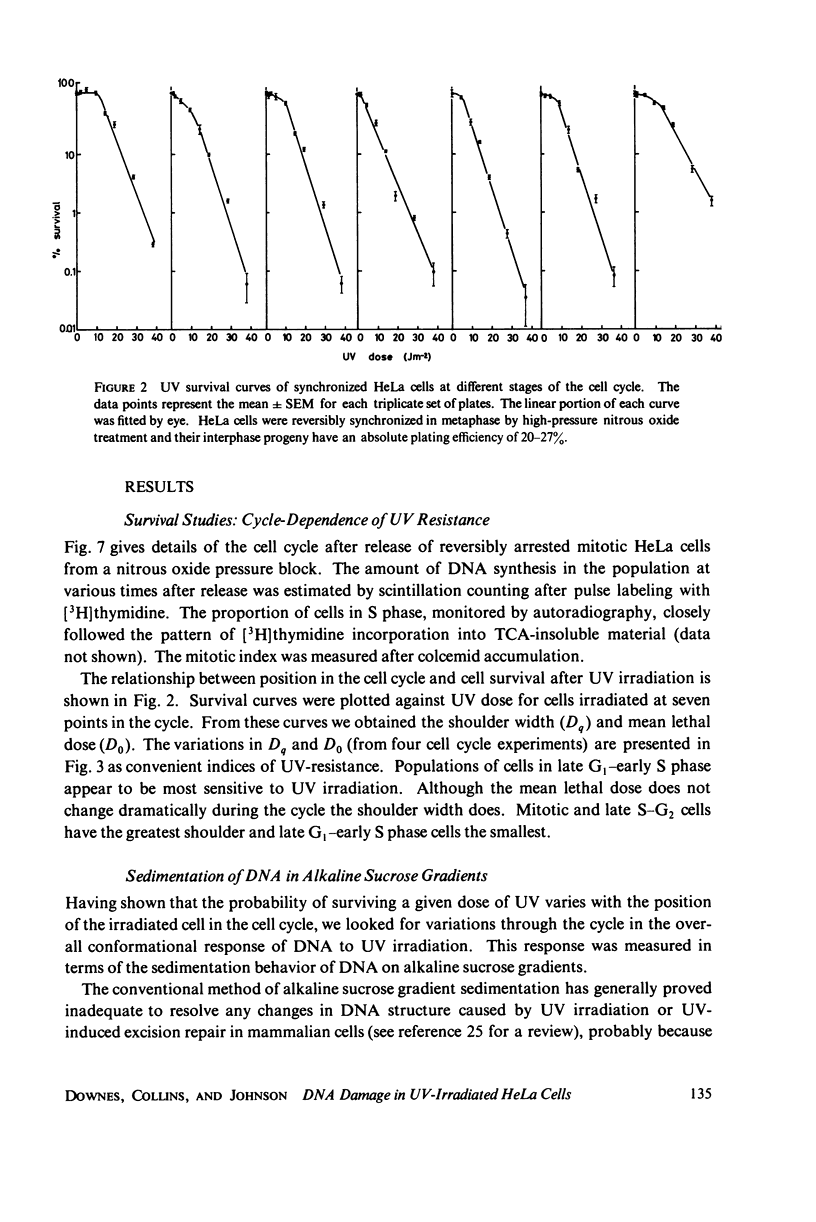
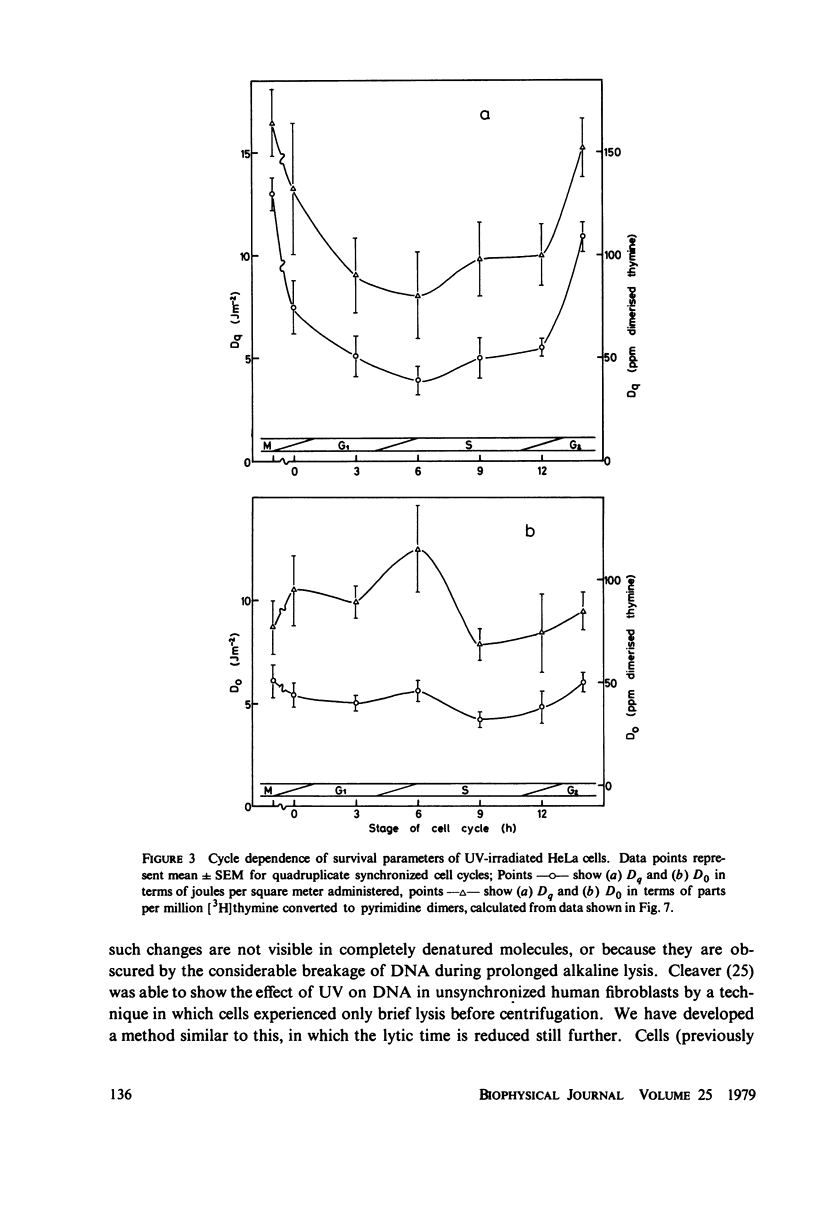
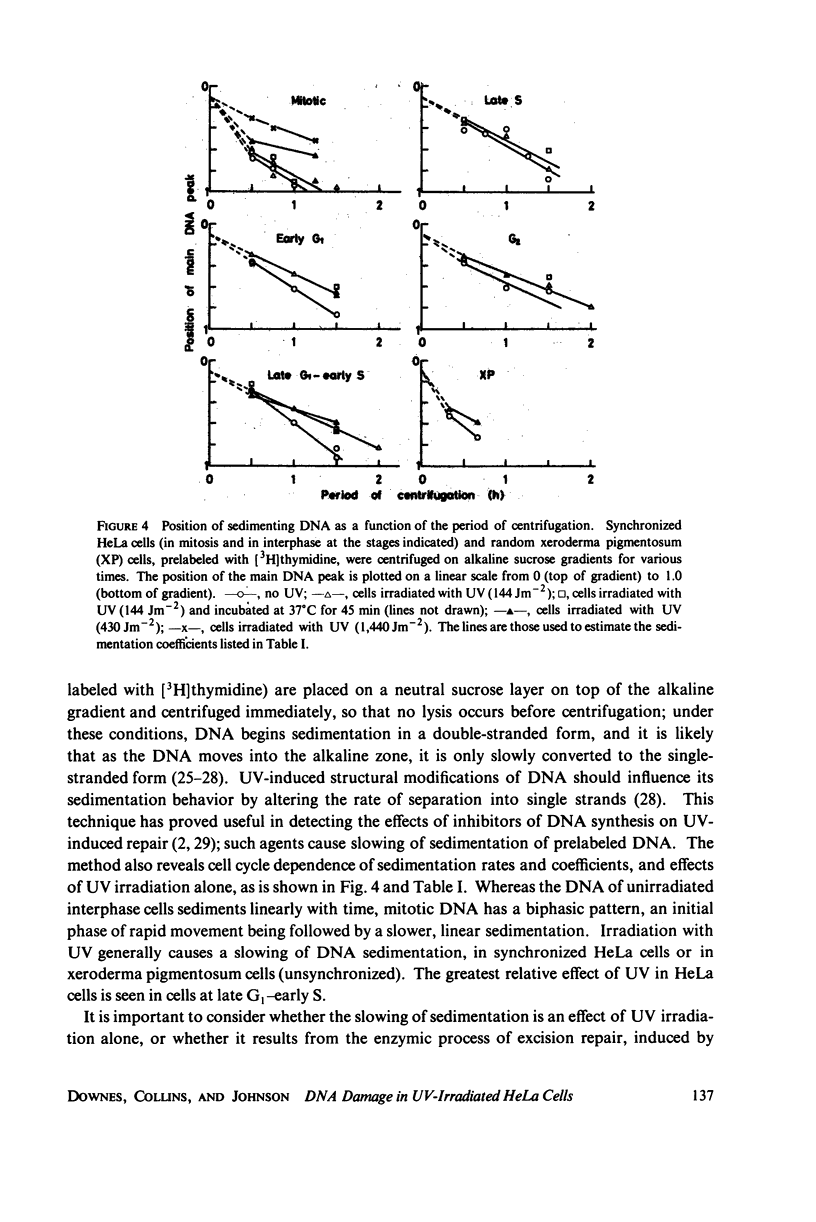
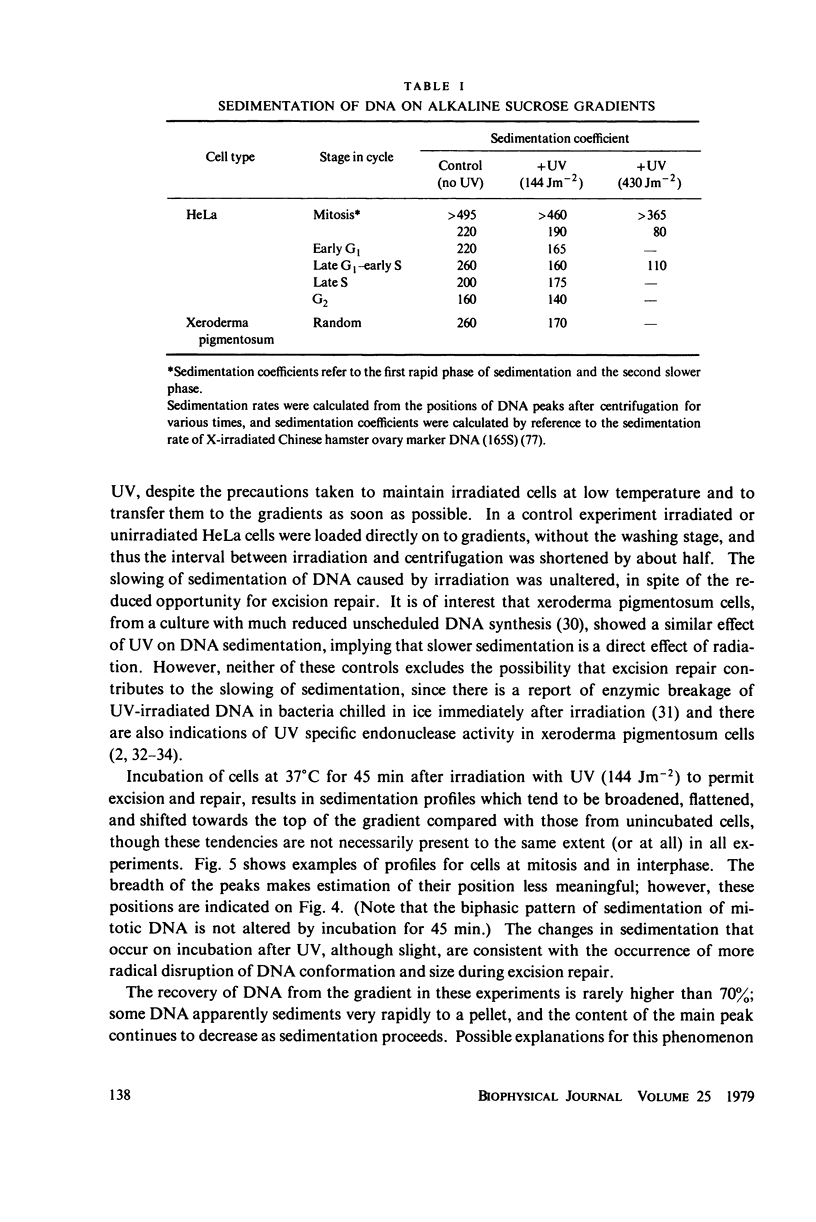
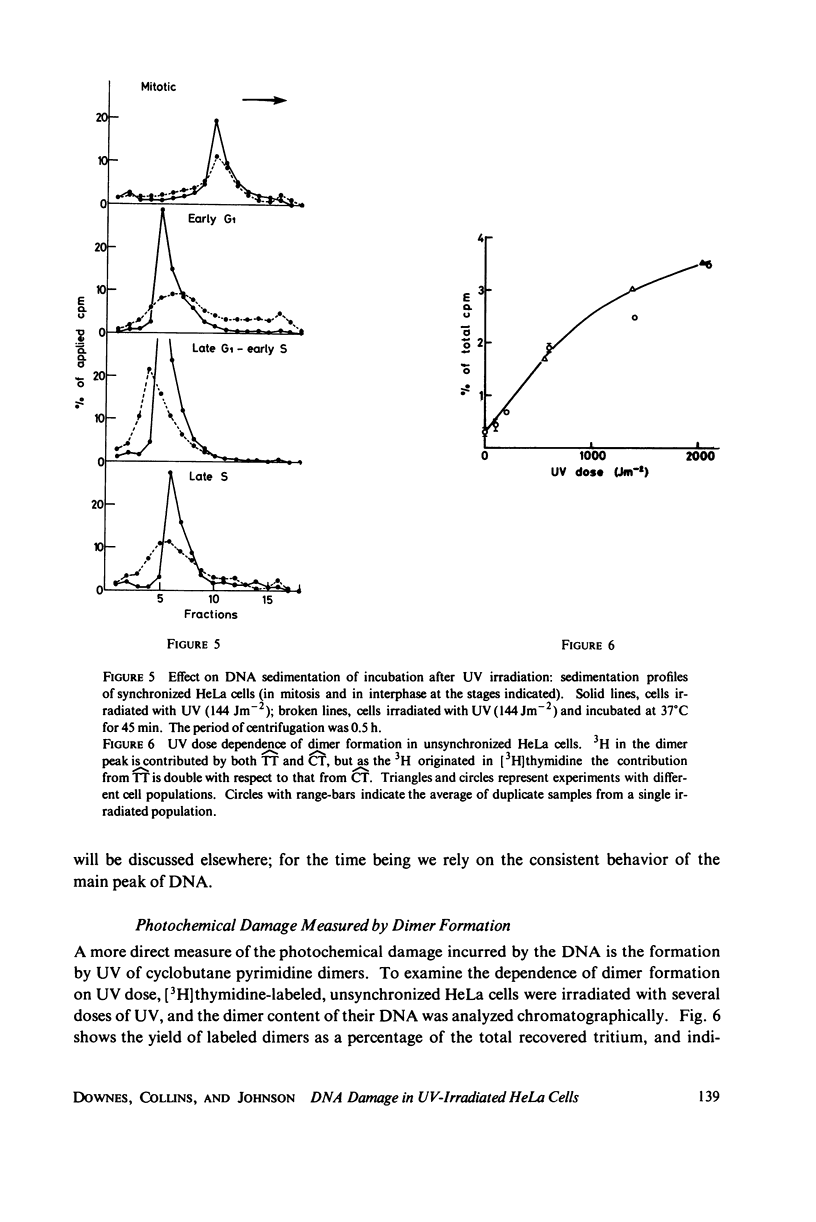
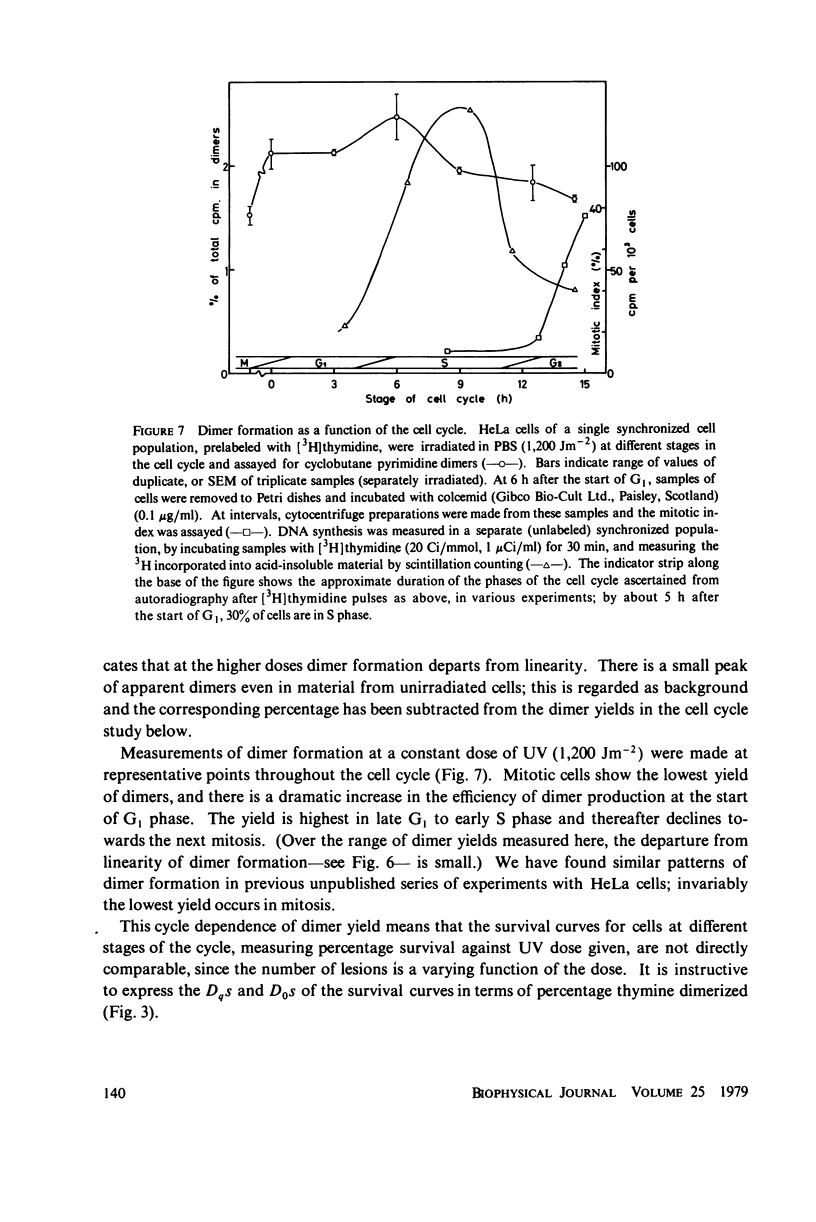
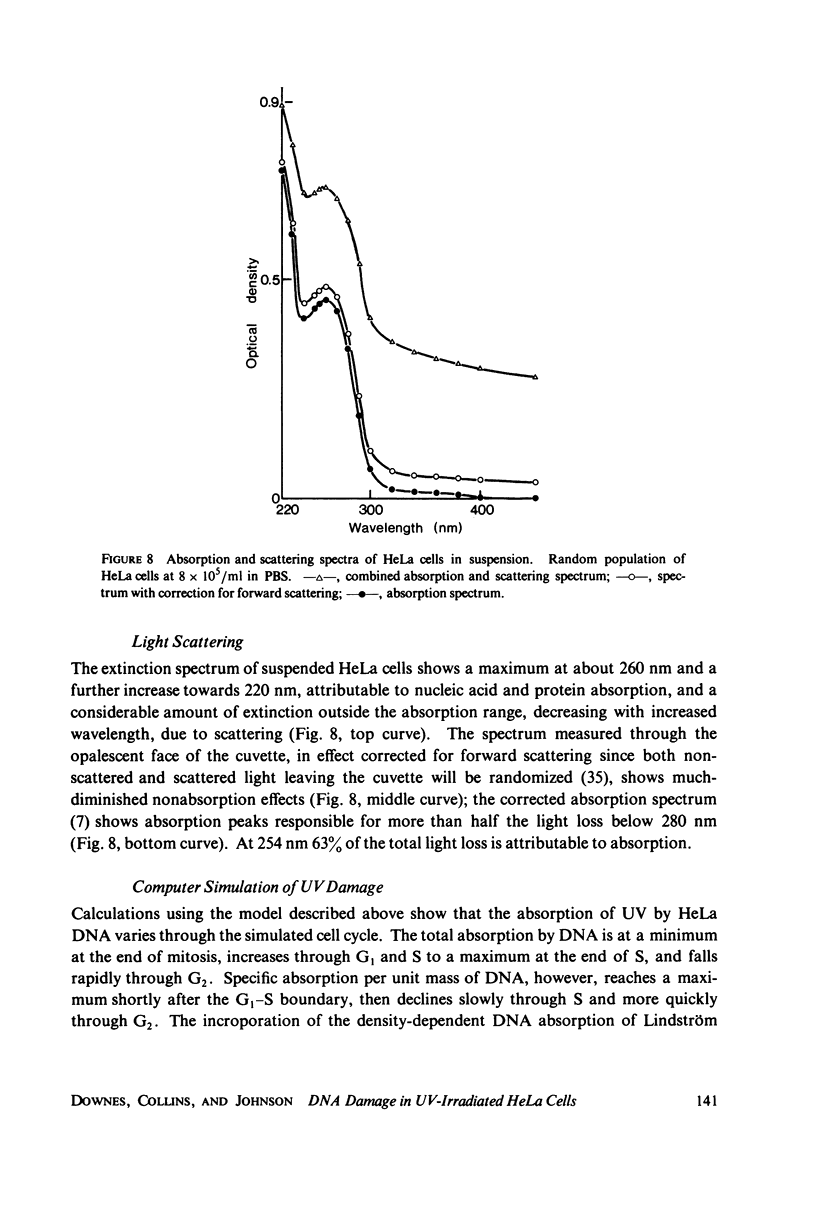
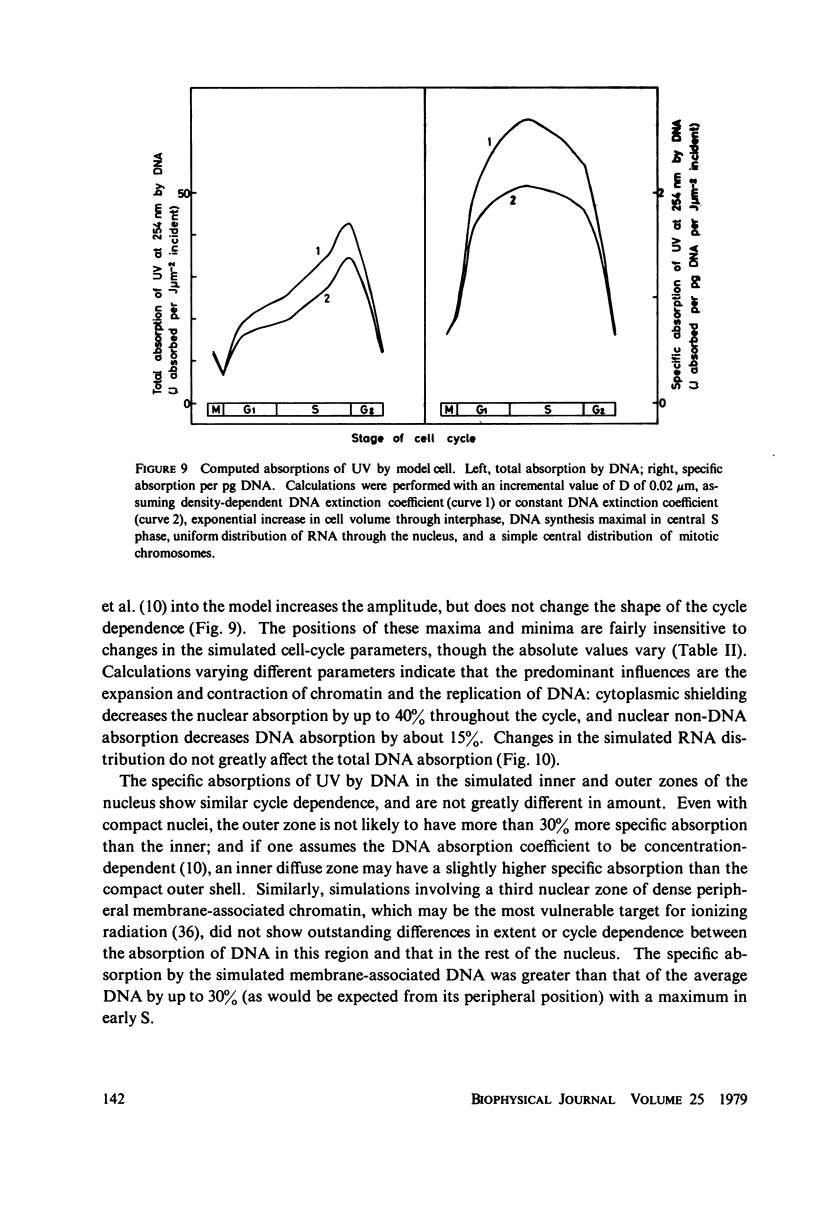
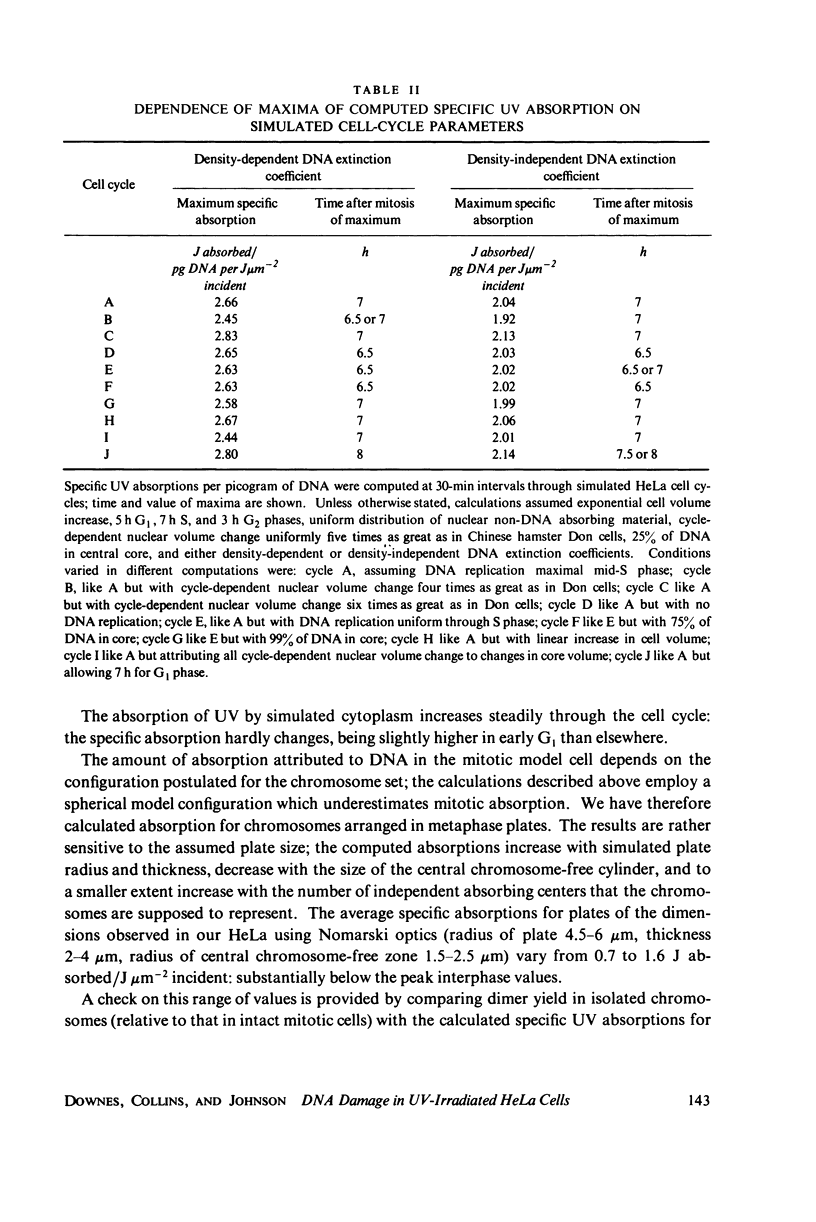
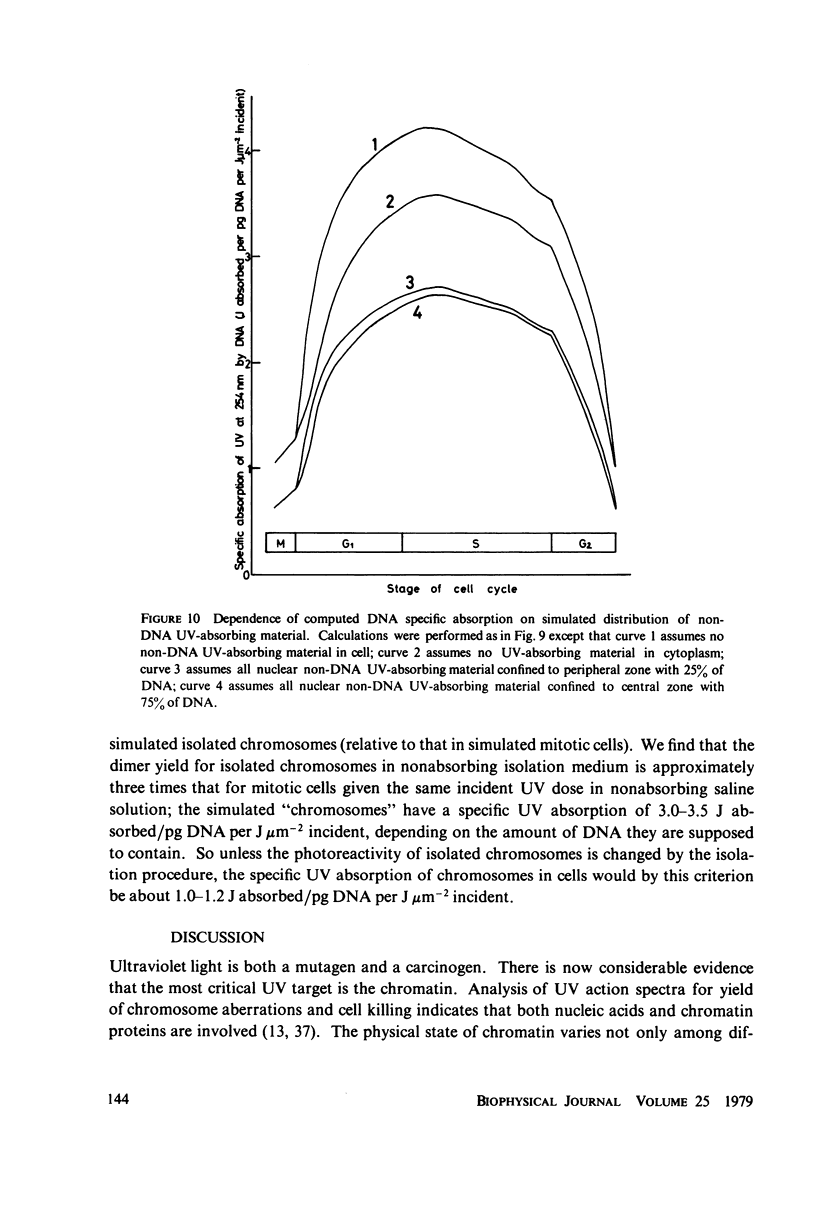
The lethal effect of UV radiation of HeLa cells is least in mitosis and greatest in late G1-early S. Photochemical damage to HeLa DNA, as measured by thymine-containing dimer formation and by alkaline sucrose sedimentation, also increases from mitosis towards early S phase. Computer simulations of UV absorption by an idealized HeLa cell at different stages of the cell cycle indicate that changes in damage could be due solely to changes in chromatin geometry. But survival is not exclusively a function of damage.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez M. R. Microfluorometric comparisons of heat-induced nuclear acridine orange metachromasia between normal cells and neoplastic cells from primary tumors of diverse origin. Cancer Res. 1975 Jan;35(1):93–98. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., van der Plas A., Veldhuisen G. A UV-specific endonucleolytic activity present in human cell extracts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):662–669. doi: 10.1016/0006-291x(72)90399-3. [DOI] [PubMed] [Google Scholar]
- Berliner J., Himes S. W., Aoki C. T., Norman A. The sites of unscheduled DNA synthesis within irradiated human lymphocytes. Radiat Res. 1975 Sep;63(3):544–552. [PubMed] [Google Scholar]
- Brent T. P. Repair enzyme suggested by mammalian endonuclease activity specific for ultraviolet-irradiated DNA. Nat New Biol. 1972 Oct 11;239(93):172–173. doi: 10.1038/newbio239172a0. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Stillman R. M., Setlow R. B., Regan J. D. DNA chain elongation and joining in normal human and xeroderma pigmentosum cells after ultraviolet irradiation. Biophys J. 1972 Sep;12(9):1183–1191. doi: 10.1016/S0006-3495(72)86154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg K., Collins A. R., Johnson R. T. Effects of ultraviolet light on synchronized Chinese hamster ovary cells; potentiation by hydroxyurea. J Cell Sci. 1977 Dec;28:29–48. doi: 10.1242/jcs.28.1.29. [DOI] [PubMed] [Google Scholar]
- Chalmers A. H., Lavin M., Atisoontornkul S., Mansbridge J., Kidson C. Resistance of human melanoma cells to ultraviolet radiation. Cancer Res. 1976 Jun;36(6):1930–1934. [PubMed] [Google Scholar]
- Chu E. H. Effects of ultraviolet radiation on mammalian cells. I. Induction of chromosome aberrations. Mutat Res. 1965 Feb;2(1):75–94. doi: 10.1016/0027-5107(65)90010-2. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Sedimentation of DNA from human fibroblasts irradiated with ultraviolet light: possible detection of excision breaks in normal and repair-deficient xeroderma pigmentosum cells. Radiat Res. 1974 Feb;57(2):207–227. [PubMed] [Google Scholar]
- Collins A. R., Schor S. L., Johnson R. T. The inhibition of repair in UV irradiated human cells. Mutat Res. 1977 Mar;42(3):413–432. doi: 10.1016/s0027-5107(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Collins J. M. Deoxyribonucleic acid structure in human diploid fibroblasts stimulated to proliferate. J Biol Chem. 1977 Jan 10;252(1):141–147. [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. DNA replication and the nuclear membrane. J Mol Biol. 1973 Apr 25;75(4):609–618. doi: 10.1016/0022-2836(73)90295-7. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Different sensitivity of DNA in situ in interphase and metaphase chromatin to heat denaturation. J Cell Biol. 1977 Apr;73(1):128–138. doi: 10.1083/jcb.73.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Gupta R., Mitra S. Strand separation of DNA induced by ultraviolet irradiation in vitro. Biochim Biophys Acta. 1974 Dec 6;374(2):145–158. doi: 10.1016/0005-2787(74)90358-x. [DOI] [PubMed] [Google Scholar]
- Dewey W. C., Noel J. S., Dettor C. M. Changes in radiosensitivity and dispersion of chromatin during the cell cycle of synchronous Chinese hamster cells. Radiat Res. 1972 Nov;52(2):373–394. [PubMed] [Google Scholar]
- Dingman C. W., Kakunaga T. DNA strand breaking and rejoining in response to ultraviolet light in normal human and xeroderma pigmentosum cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Jul;30(1):55–66. doi: 10.1080/09553007614550801. [DOI] [PubMed] [Google Scholar]
- Djordjevic B., Tolmach L. J. Responses of synchronous populations of HeLa cells to ultraviolet irradiation at selected stages of the generation cycle. Radiat Res. 1967 Oct;32(2):327–346. [PubMed] [Google Scholar]
- Duker N. J., Teebor G. W. Different ultraviolet DNA endonuclease activity in human cells. Nature. 1975 May 1;255(5503):82–84. doi: 10.1038/255082a0. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Griggs H. G., Bender M. A. Ultraviolet and gamma-ray induced reproductive death and photoreactivation in a Xenopus tissue culture cell line. Photochem Photobiol. 1972 Jun;15(6):517–526. doi: 10.1111/j.1751-1097.1972.tb06264.x. [DOI] [PubMed] [Google Scholar]
- Han A., Korbelik M., Ban J. DNA-to-protein cross-linking in synchronized HeLa cells exposed to ultra-violet light. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Jan;27(1):63–74. doi: 10.1080/09553007514550061. [DOI] [PubMed] [Google Scholar]
- Han A., Sinclair W. K. Sensitivity of synchronized Chinese hamster cells to ultraviolet light. Biophys J. 1969 Sep;9(9):1171–1192. doi: 10.1016/S0006-3495(69)86444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Connor R. J., Jackson F. E., Lieberman M. W. Intranuclear distribution of DNA repair synthesis induced by chemical carcinogens or ultraviolet light in human diploid fibroblasts. Cancer Res. 1974 Dec;34(12):3461–3468. [PubMed] [Google Scholar]
- Hildebrand C. E., Tobey R. A. Cell-cycle-specific changes in chromatin organization. Biochem Biophys Res Commun. 1975 Mar 3;63(1):134–139. doi: 10.1016/s0006-291x(75)80021-0. [DOI] [PubMed] [Google Scholar]
- Hsu T. C. A possible function of constitutive heterochromatin: the bodyguard hypothesis. Genetics. 1975 Jun;79 (Suppl):137–150. [PubMed] [Google Scholar]
- Ichihashi M., Ramsay C. A. The action spectrum and dose response studies of unscheduled DNA synthesis in normal human fibroblasts. Photochem Photobiol. 1976 Feb;23(2):103–106. doi: 10.1111/j.1751-1097.1976.tb06780.x. [DOI] [PubMed] [Google Scholar]
- Isomura K., Nikaido O., Horikawa M., Sugahara T. Repair of DNA damage in ultraviolet-sensitive cells isolated from HeLa S3 cells. Radiat Res. 1973 Jan;53(1):143–152. [PubMed] [Google Scholar]
- Jolley G. M., Ormerod M. G. The incomplete separation of complementary strands of high molecular weight DNA in alkali. Biochim Biophys Acta. 1974 Jun 27;353(2):200–214. doi: 10.1016/0005-2787(74)90185-3. [DOI] [PubMed] [Google Scholar]
- KILLANDER D., ZETTERBERG A. QUANTITATIVE CYTOCHEMICAL STUDIES ON INTERPHASE GROWTH. I. DETERMINATION OF DNA, RNA AND MASS CONTENT OF AGE DETERMINED MOUSE FIBROBLASTS IN VITRO AND OF INTERCELLULAR VARIATION IN GENERATION TIME. Exp Cell Res. 1965 May;38:272–284. doi: 10.1016/0014-4827(65)90403-9. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Painter R. B. Multiple thymidine incorporation peaks in the S phase of synchronous human diploid fibroblasts. Exp Cell Res. 1977 Jul;107(2):428–431. doi: 10.1016/0014-4827(77)90364-0. [DOI] [PubMed] [Google Scholar]
- Karn J., Johnson E. M., Vidali G., Allfrey V. G. Differential phosphorylation and turnover of nuclear acidic proteins during the cell cycle of synchronized HeLa cells. J Biol Chem. 1974 Feb 10;249(3):667–677. [PubMed] [Google Scholar]
- LATIMER P., EUBANKS C. A. Absorption spectrophotometry of turbid suspensions: a method of correcting for large systematic distortions. Arch Biochem Biophys. 1962 Aug;98:274–285. doi: 10.1016/0003-9861(62)90184-4. [DOI] [PubMed] [Google Scholar]
- Lang H., Luck G. Ultraviolet-light-induced conformational changes in DNA. Photochem Photobiol. 1973 Jun;17(6):387–393. doi: 10.1111/j.1751-1097.1973.tb06372.x. [DOI] [PubMed] [Google Scholar]
- Lindström M., Zetterberg A., Carlson L. Quantitative microspectrophotometric analysis of nucleic acids in concentrated solutions, in solid droplets and in lymphocytes. Exp Cell Res. 1966 Oct;43(3):537–545. doi: 10.1016/0014-4827(66)90024-3. [DOI] [PubMed] [Google Scholar]
- Linn J. D., Wheeler K. T. Alkali unwinding kinetics of mammalian DNA in a simulated viscoelastometry experiment. Biochem Biophys Res Commun. 1975 Sep 16;66(2):712–716. doi: 10.1016/0006-291x(75)90568-9. [DOI] [PubMed] [Google Scholar]
- MARMUR J., ANDERSON W. F., MATTHEWS L., BERNS K., GAJEWSKA E., LANE D., DOTY P. The effects of ultraviolet light on the biological and physical chemical properties of deoxyribonucleic acids. J Cell Comp Physiol. 1961 Dec;58(3):33–55. doi: 10.1002/jcp.1030580406. [DOI] [PubMed] [Google Scholar]
- MELLORS R. C., BERGER R. E., STREIM H. G. Ultraviolet microscopy and microspectroscopy of resting and dividing cells studies with a reflecting microscope. Science. 1950 Jun 9;111(2893):627–632. doi: 10.1126/science.111.2893.627. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brunsting A. Light scattering from nucleated biological cells. Biophys J. 1975 Mar;15(3):191–203. doi: 10.1016/S0006-3495(75)85811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini C., Ajiro K., Borun T. W., Baserga R. Chromatin changes during the cell cycle of HeHa cells. J Biol Chem. 1975 May 10;250(9):3381–3385. [PubMed] [Google Scholar]
- Okubo S., Nakayama H., Takagi Y. Repair of ultraviolet-damaged DNA in Micrococcus lysodeikticus. II. In vivo investigation on endonuclease activity specific for ultraviolet-irradiated DNA. Biochim Biophys Acta. 1971 Jan 1;228(1):83–94. [PubMed] [Google Scholar]
- Pederson T. Chromatin structure and the cell cycle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2224–2228. doi: 10.1073/pnas.69.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Robbins E. Chromatin structure and the cell division cycle. Actinomycin binding in synchronized HeLa cells. J Cell Biol. 1972 Nov;55(2):322–327. doi: 10.1083/jcb.55.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P. N. Mitotic synchrony in mammalian cells treated with nitrous oxide at high pressure. Science. 1968 May 17;160(3829):774–776. doi: 10.1126/science.160.3829.774. [DOI] [PubMed] [Google Scholar]
- Rauth A. M., Tammemagi M., Hunter G. Nascent DNA synthesis in ultraviolet light-irradiated mouse, human and Chinese hamster cells. Biophys J. 1974 Mar;14(3):209–220. doi: 10.1016/S0006-3495(74)85908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth A. M., Whitmore G. F. The survival of synchronized L cells after ultraviolet irradiation. Radiat Res. 1966 May;28(1):84–95. [PubMed] [Google Scholar]
- Rydberg B. The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat Res. 1975 Feb;61(2):274–287. [PubMed] [Google Scholar]
- SINCLAIR W. K., MORTON R. A. X-RAY AND ULTRAVIOLET SENSITIVITY OF SYNCHRONIZED CHINESE HAMSTER CELLS AT VARIOUS STAGES OF THE CELL CYCLE. Biophys J. 1965 Jan;5:1–25. doi: 10.1016/s0006-3495(65)86700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUBBLEFIELD E., MUELLER G. C. Molecular events in the reproduction of animal cells. II. The focalized synthesis of DNA in the chromosomes of HeLa cells. Cancer Res. 1962 Oct;22:1091–1099. [PubMed] [Google Scholar]
- Salganik R. I., Drevich V. F., Vasyunina E. A. Isolation of ultraviolet-denatured regions of DNA and their base composition. J Mol Biol. 1967 Nov 28;30(1):219–222. doi: 10.1016/0022-2836(67)90255-0. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Johnson R. T., Waldren C. A. Changes in the organization of chromosomes during the cell cycle: response to ultraviolet light. J Cell Sci. 1975 May;17(3):539–565. doi: 10.1242/jcs.17.3.539. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Yasuda S., Okubo S., Nakayama H., Shimada K., Takagi Y. Mechanism of repair of DNA in bacteriophage. I. Excision of pyrimidine dimers from ultraviolet-irradiated DNA by an extract of T4-infected cells. J Mol Biol. 1970 Jan 28;47(2):231–242. doi: 10.1016/0022-2836(70)90342-6. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Stafford R. S., Allison D. P., Rahn R. O. Detection by electron microscopy of photo-induced denaturation in lambda DNA. Nucleic Acids Res. 1975 Feb;2(2):143–148. doi: 10.1093/nar/2.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward D. L., Humphrey R. M. Induction of thymine dimers in synchronized populations of Chinese hamster cells. Nature. 1966 Oct 15;212(5059):298–300. doi: 10.1038/212298b0. [DOI] [PubMed] [Google Scholar]
- Todd P., Dalen H., Schroy C. B. Survival of synchronized cultured human liver cells following single and fractionated exposures to ultraviolet light. Radiat Res. 1977 Mar;69(3):573–582. [PubMed] [Google Scholar]
- Watanabe M., Horikawa M. Analyses of differential sensitivities of synchronized HeLa S 3 cells to radiations and chemical carcinogen during the cell cycle. 1. Establishment of optimum conditions for obtaining a large purified synchronized population. J Radiat Res. 1973 Sep;14(3):258–270. doi: 10.1269/jrr.14.258. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Horikawa M. Analysis of differential sensitivities of synchronized HeLa S3 cells to radiations and chemical carcinogen during the cell cycle. II. Ultraviolet light. Biochem Biophys Res Commun. 1974 May 7;58(1):185–191. doi: 10.1016/0006-291x(74)90909-7. [DOI] [PubMed] [Google Scholar]
- Weinblum D., Breter H. J., Zahn R. K., Berger J. Alteration of DNA reassociation kinetics due to base mismatch caused by thymine dimerisation. Biochim Biophys Acta. 1974 Dec 20;374(3):324–331. doi: 10.1016/0005-2787(74)90253-6. [DOI] [PubMed] [Google Scholar]
- Wheeler K. T., DeWitt J., Lett J. T. A marker for mammalian DNA sedimentation. Radiat Res. 1974 Mar;57(3):365–378. [PubMed] [Google Scholar]
- Williams J. R., Little J. B. Selective protection of cultured human cells from the toxic effects of ultraviolet light by proflavine pretreatment. Radiat Res. 1977 Oct;72(1):154–163. [PubMed] [Google Scholar]
- Wray W. Isolation of metaphase chromosomes, mitotic apparatus, and nuclei. Methods Cell Biol. 1973;6:283–306. doi: 10.1016/s0091-679x(08)60053-9. [DOI] [PubMed] [Google Scholar]
- Zermeno A., Cole A. Radiosensitive structure of metaphase and interphase hamster cells as studies by low-voltage electron beam irradiation. Radiat Res. 1969 Sep;39(3):669–684. [PubMed] [Google Scholar]
- Zetterberg A. Nuclear and cytoplasmic nucleic acid content and cytoplasmic protein synthesis during interphase in mouse fibroblasts in vitro. Exp Cell Res. 1966 Oct;43(3):517–525. doi: 10.1016/0014-4827(66)90022-x. [DOI] [PubMed] [Google Scholar]


