Abstract
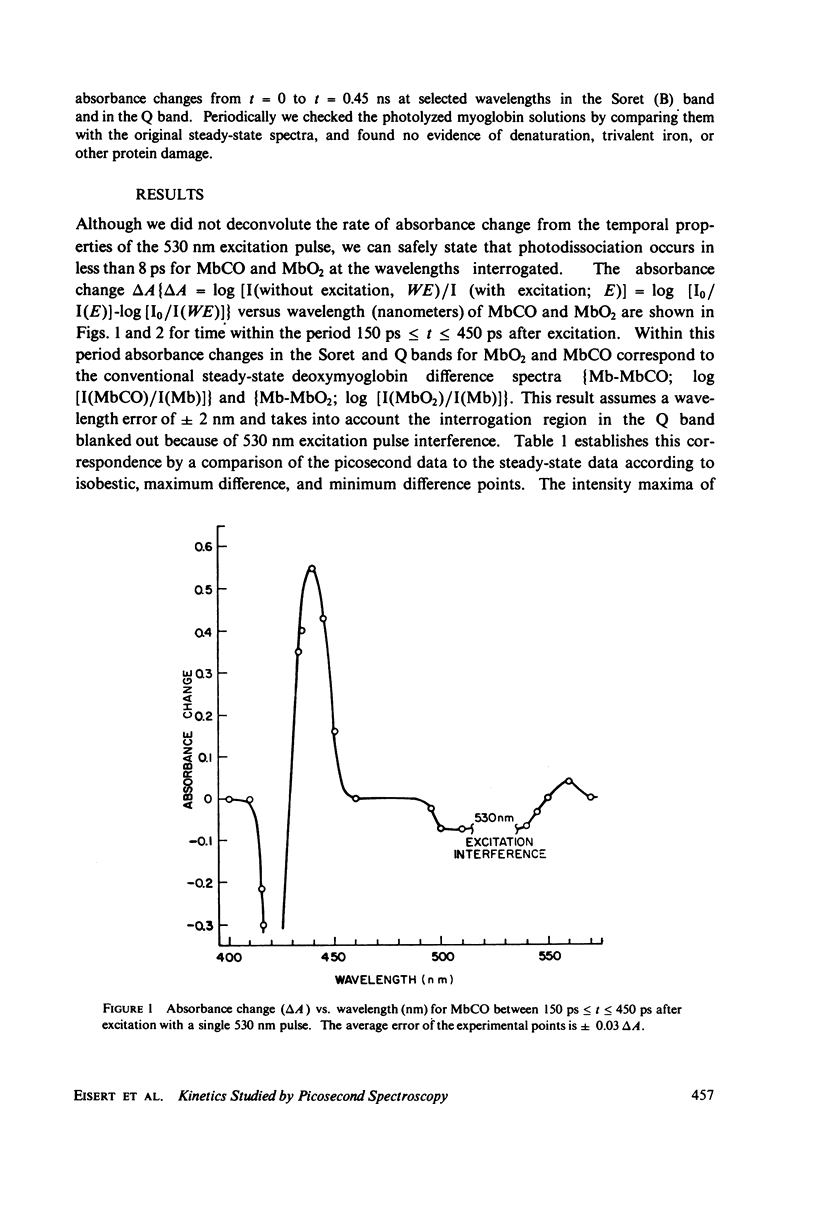
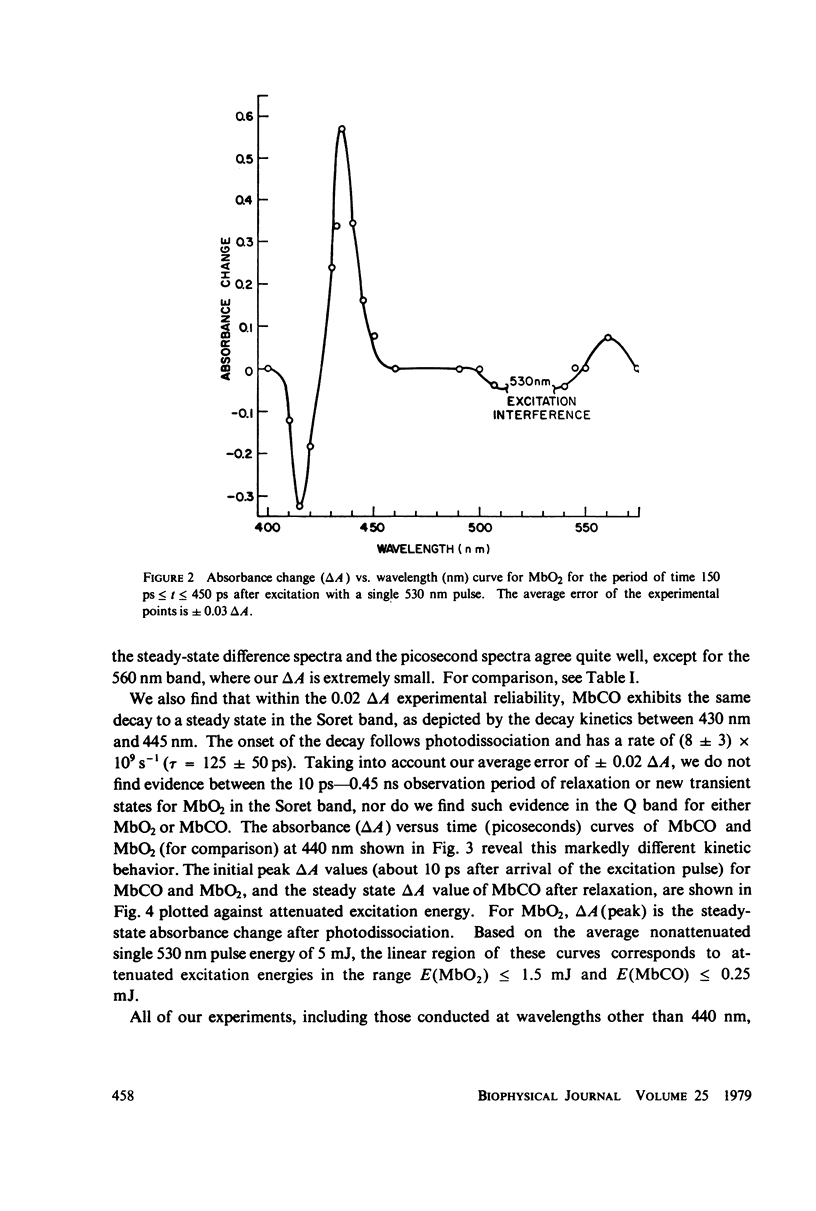
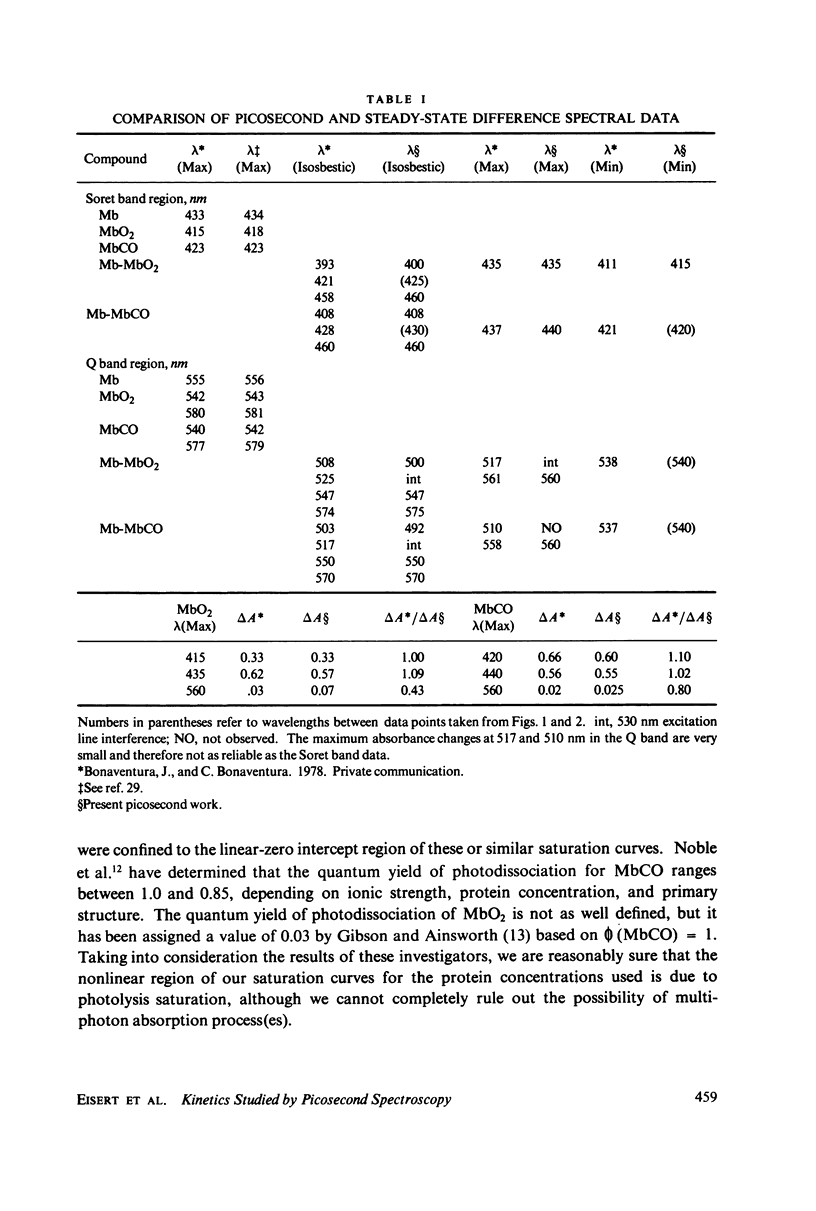
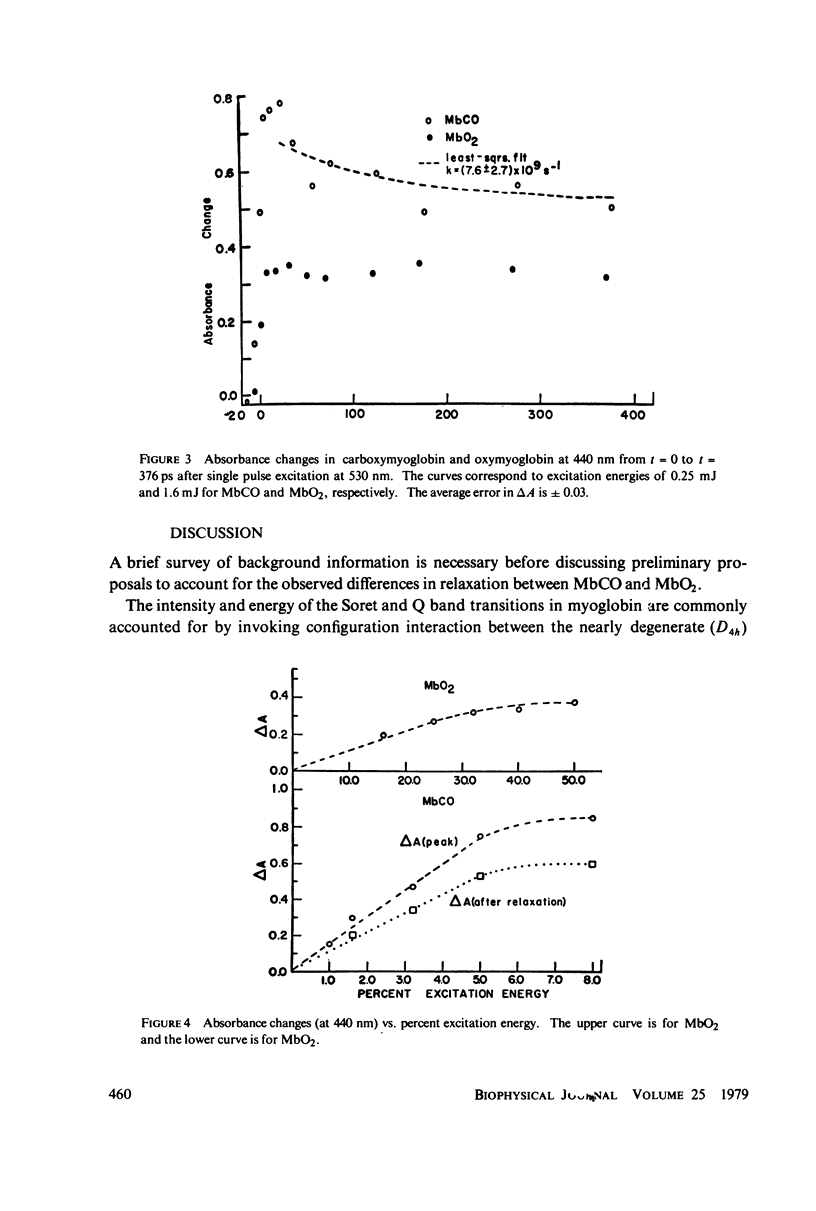
Picosecond studies of carboxymyoglobin (MbCO) and oxymyoglobin (MbO2) reveal that excitation at 530 nm induces photodissociation at less than 8 ps. The kinetic and structural changes were monitored by following absorbance changes at selected wave-lengths in the Soret (B) band and in the Q band. Within the 10 ps-0.45 ns period of time over which our experiments were conducted, the absorbance changes in the Soret and Q bands for MbCO and MbO2 correspond to the conventional long-term, steady-state deoxymyoglobin difference spectra (Mb-MbCO and Mb-MbO2), as determined by comparison of isosbestic, maximum, and minimum points. In addition, MbCO exhibits a decay to a steady state in the Soret band (monitored at 440 nm). The onset of the decay immediately follows photodissociation and has a rate of (8 +/- 3) X 10(9) s-1 (tau = 125 +/- 50 ps). During the 10 ps-0.45 ns observation window, relaxation is not seen for MbO2 in the Soret band, nor is relaxation observed in the Q band for either MbCO or MbO2. We conclude from these results that the steady state that we observed for MbCO and MbO2 is most likely the stable form of deoxymyoglobin, and the relaxational differences between MbCO and MbO2 observed in the Soret band indicate that the electronic destabilization after ligand detachment is very different for these molecules. We believe that these relaxational differences may be related to differences in tertiary structural changes, or due to the fact that the MbCO (S = 0) molecule passes through an intermediate spin Mb (S = 1) state before relaxing the the Mb (S = 2) state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert B., Banerjee R., Lindqvist L. The kinetics of conformational changes in hemoglobin, studied by laser photolysis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):558–562. doi: 10.1073/pnas.71.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C., Pacyna B. The conversion of trivalent to divalent iron in hemoglobin of various species. Anal Biochem. 1975 May 12;65(1-2):445–448. doi: 10.1016/0003-2697(75)90531-x. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Cooperative interactions of hemoglobin. Annu Rev Biochem. 1975;44:209–232. doi: 10.1146/annurev.bi.44.070175.001233. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., AINSWORTH S. Photosensitivity of haem compounds. Nature. 1957 Dec 21;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- Goddard W. A., 3rd, Olafson B. D. Ozone model for bonding of an O2 to heme in oxyhemoglobin. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2335–2339. doi: 10.1073/pnas.72.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Lang G., Marshall W. Mössbauer effect in some haemoglobin compounds. J Mol Biol. 1966 Jul;18(3):385–404. doi: 10.1016/s0022-2836(66)80032-3. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Optically detected conformational changes in haemoglobin single crystals. Nature. 1974 Jan 4;247(5435):62–64. doi: 10.1038/247062a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Polarized single crystal absorption spectra of carboxy- and oxyhemoglobin. Ann N Y Acad Sci. 1973;206:210–222. doi: 10.1111/j.1749-6632.1973.tb43213.x. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Brunori M., Wyman J., Antonini E. Studies on the quantum yields of the photodissociation of carbon monoxide from hemoglobin and myoglobin. Biochemistry. 1967 Apr;6(4):1216–1222. doi: 10.1021/bi00856a035. [DOI] [PubMed] [Google Scholar]
- Noe L. J., Eisert W. G., Rentzepis P. M. Picosecond photodissociation and subsequent recombination processes in carbon monoxide hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):573–577. doi: 10.1073/pnas.75.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson B. D., Goddard W. A., 3rd Molecular description of dioxygen bonding in hemoglobin. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1315–1319. doi: 10.1073/pnas.74.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. Magnetic properties and structure of oxyhemoglobin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2612–2613. doi: 10.1073/pnas.74.7.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Shank C. V., Ippen E. P., Bersohn R. Time-resolved spectroscopy of hemoglobin and its complexes with subpicosecond optical pulses. Science. 1976 Jul 2;193(4247):50–51. doi: 10.1126/science.935853. [DOI] [PubMed] [Google Scholar]
- Trautwein A., Eicher H., Mayer A., Alfsen A., Waks M., Rosa J., Beuzard Y. Modification of the electronic structure of ferrous iron in hemoglobin by ligandation and by alterations of the protein structure inferred from Mössbauer measurements. J Chem Phys. 1970 Aug 1;53(3):963–967. doi: 10.1063/1.1674164. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- Weissbluth M., Maling J. E. Interpretation of quadrupole splittings and isomer shifts in hemoglobin. J Chem Phys. 1967 Nov 15;47(10):4166–4172. doi: 10.1063/1.1701594. [DOI] [PubMed] [Google Scholar]


