Abstract
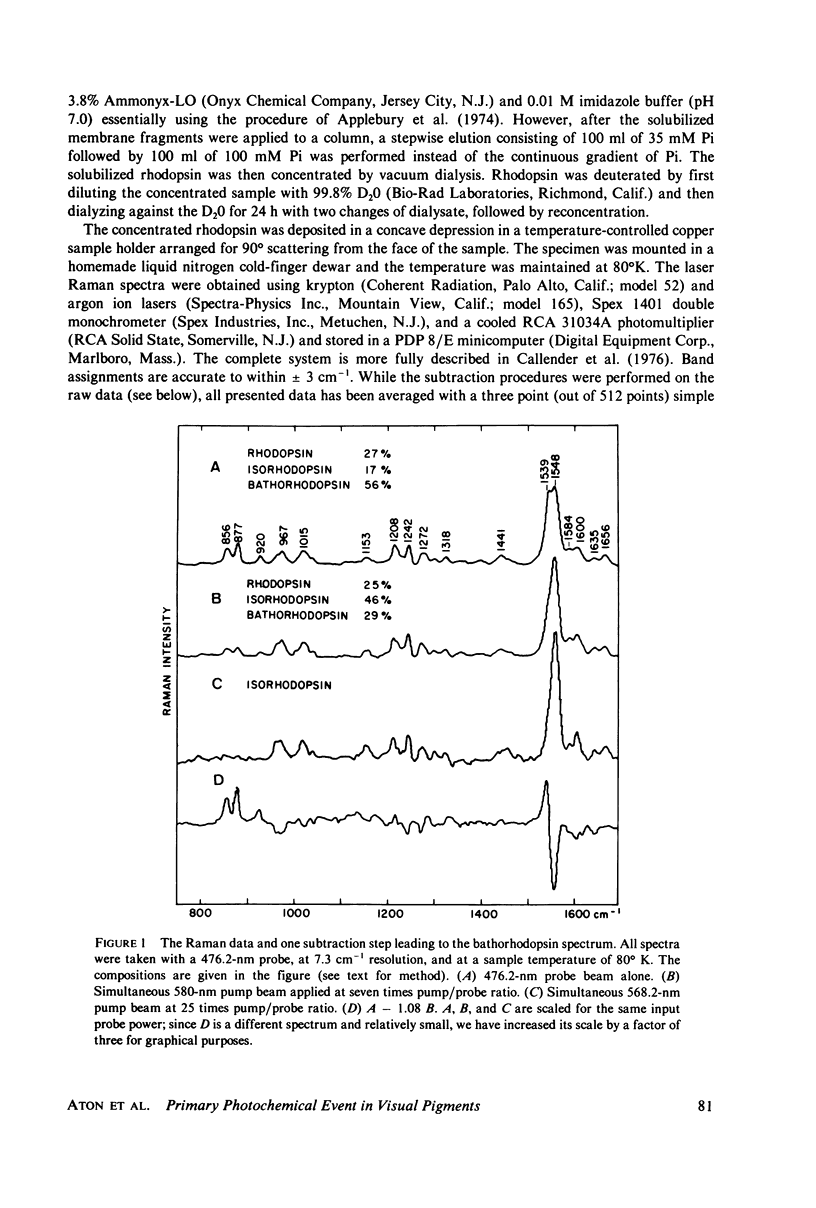
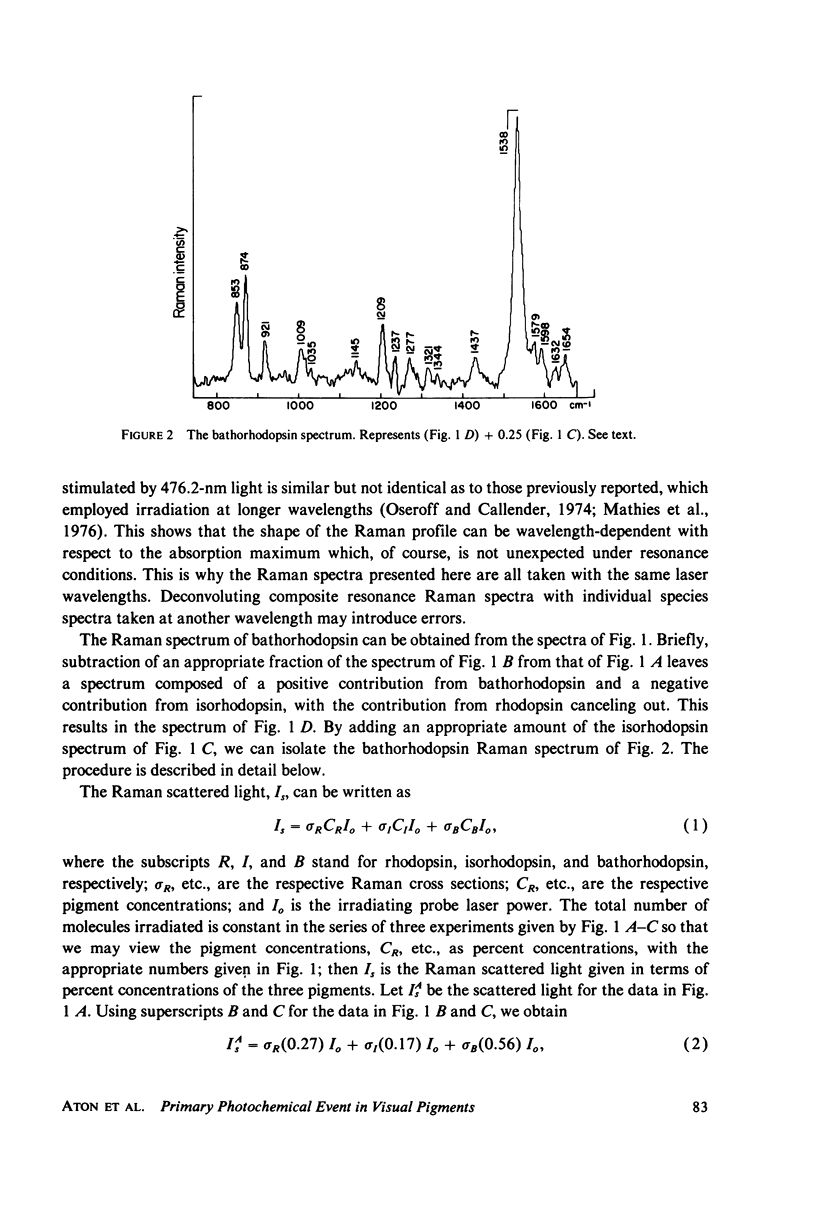
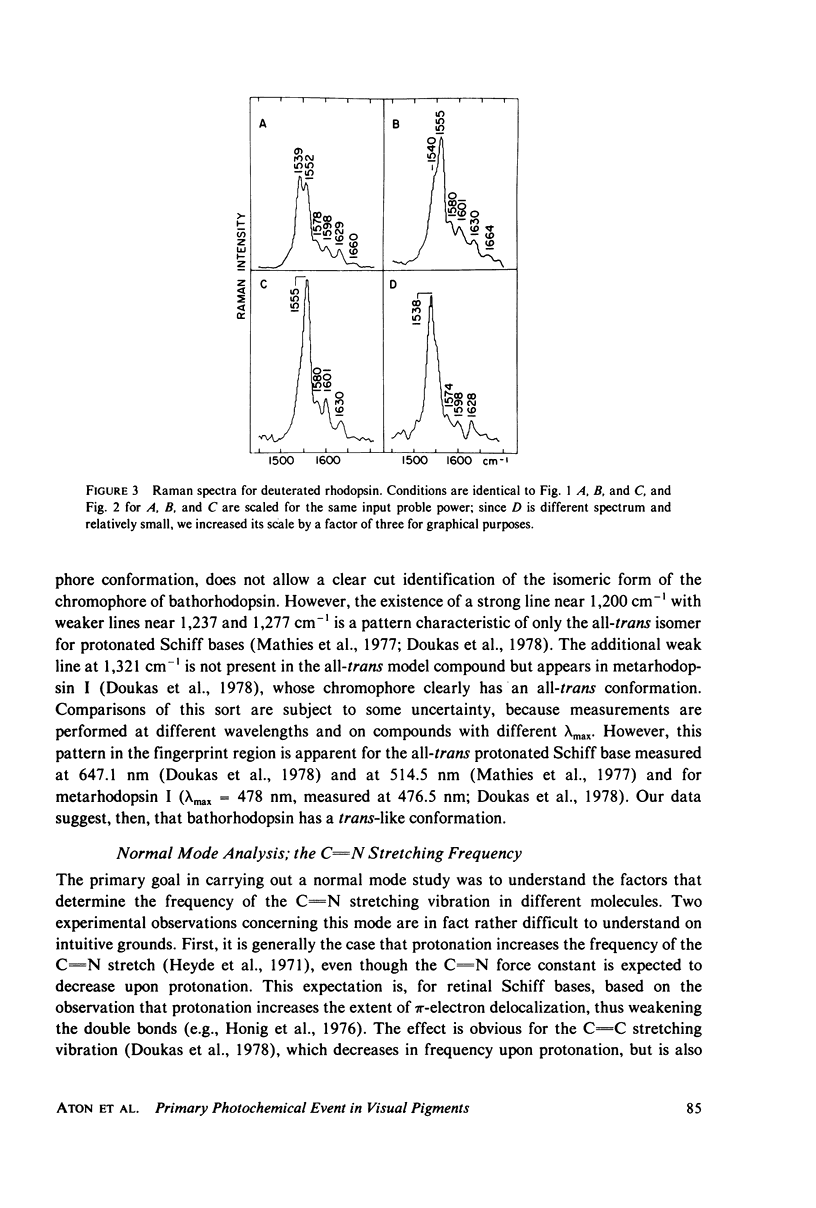
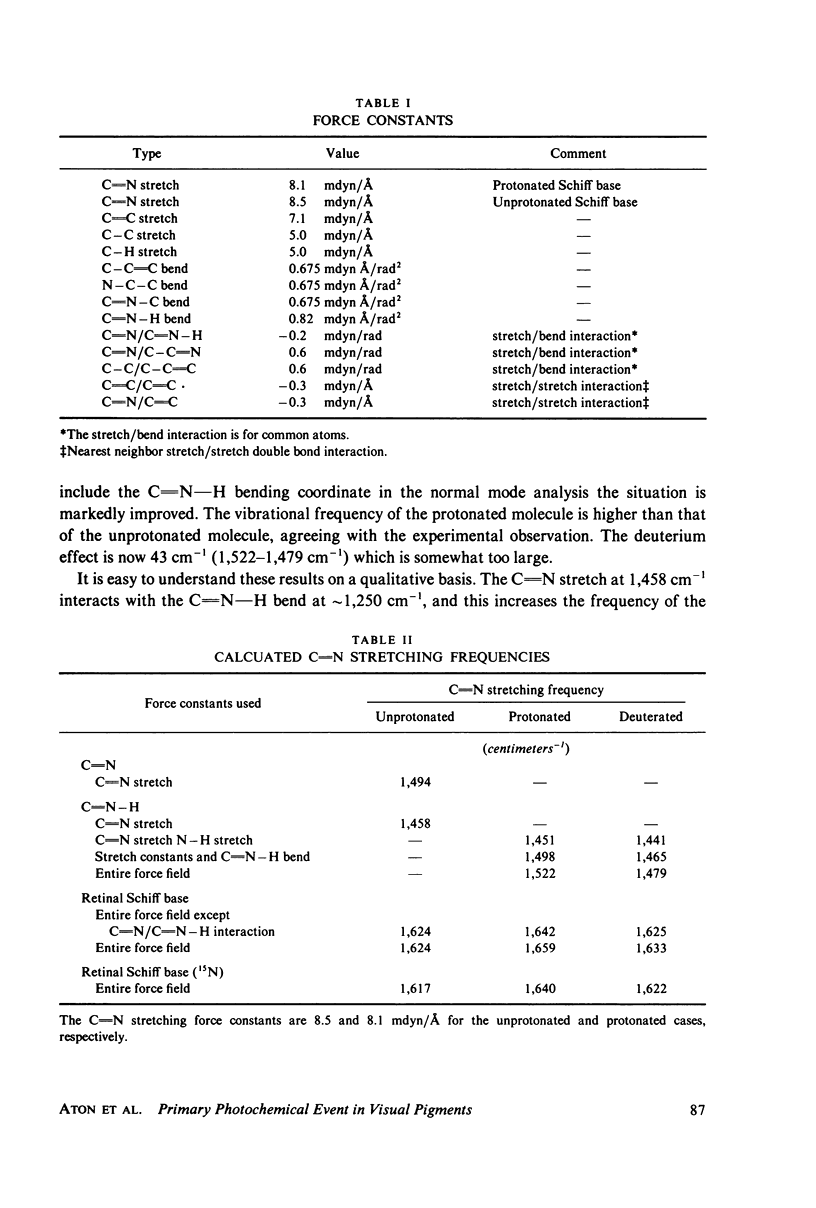
Resonance Raman multicomponent spectra of bovine rhodopsin, isorhodopsin, and bathorhodopsin have been obtained at low temperature. Application of the double beam "pump-probe" technique allows us to extract a complete bathorhodopsin spectrum from the mixture in both protonated and deuterated media. Our results show that the Schiff base of bathorhodopsin is fully protonated and that the extent of protonation is unaffected by its photochemical formation from either rhodopsin or isorhodopsin. The Raman spectrum of bathorhodopsin is significantly different than that of either parent pigment, thus supporting the notion that a geometric change in the chromophore is an important component of the primary photochemical event in vision. A normal mode analysis is carried out with particular attention devoted to the factors that determine the frequency of the C=N stretching vibration. We find that the increased frequency of this mode in protonated relative to unprotonated Schiff bases is due to coupling between C=N stretching and C=N-H bending motions, and the shift observed upon deuteration of the Schiff base can also be understood in these terms. Various models for the primary event are discussed in light of our experimental and theoretical results.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Peters K. S., Rentzepis P. M. Primary intermediates in the photochemical cycle of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):375–382. doi: 10.1016/S0006-3495(78)85456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Aton B., Callender R. H., Honig B. Photochemical cis-trans isomerisation of bovine rhodopsin at liquid helium temperatures. Nature. 1978 Jun 29;273(5665):784–786. doi: 10.1038/273784a0. [DOI] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H. Effect of selected anions and solvents on the electron absorption, nuclear magnetic resonance, and infrared spectra of the N-retinylidene-n-butylammonium cation. Biochemistry. 1975 Jun 3;14(11):2304–2309. doi: 10.1021/bi00682a005. [DOI] [PubMed] [Google Scholar]
- Callender R. H., Doukas A., Crouch R., Nakanishi K. Molecular flow resonance Raman effect from retinal and rhodopsin. Biochemistry. 1976 Apr 20;15(8):1621–1629. doi: 10.1021/bi00653a005. [DOI] [PubMed] [Google Scholar]
- Callender R. Resonance Raman studies of visual pigments. Annu Rev Biophys Bioeng. 1977;6:33–55. doi: 10.1146/annurev.bb.06.060177.000341. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. The use of Ammonyx LO in the purification of rhodopsin and rod outer segments. Vision Res. 1971 Sep;11(9):1007–1009. doi: 10.1016/0042-6989(71)90220-3. [DOI] [PubMed] [Google Scholar]
- Eyring G., Mathies R. Resonance Raman studies of bathorhodopsin: evidence for a protonated Schiff base linkage. Proc Natl Acad Sci U S A. 1979 Jan;76(1):33–37. doi: 10.1073/pnas.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde M. E., Gill D., Kilponen R. G., Rimai L. Raman spectra of Schiff bases of retinal (models of visual photoreceptors). J Am Chem Soc. 1971 Dec 15;93(25):6776–6780. doi: 10.1021/ja00754a012. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T. G. The structure and spectra of the chromophore of the visual pigments. Annu Rev Biophys Bioeng. 1974;3(0):151–177. doi: 10.1146/annurev.bb.03.060174.001055. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I., Favrot J., Leclercq J. M., Vocelle D., Sándorfy C. Photochemistry of visual pigments: an interpretation of spectral changes in terms of molecular associations and isomerization. Rev Can Biol. 1978 Dec;37(4):257–271. [PubMed] [Google Scholar]
- Mathies R., Freedman T. B., Stryer L. Resonance Raman studies of the conformation of retinal in rhodopsin and isorhodopsin. J Mol Biol. 1977 Jan 15;109(2):367–372. doi: 10.1016/s0022-2836(77)80040-5. [DOI] [PubMed] [Google Scholar]
- Mathies R., Oseroff A. R., Stryer L. Rapid-flow resonance Raman spectroscopy of photolabile molecules: rhodopsin and isorhodopsin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):1–5. doi: 10.1073/pnas.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabolt J. F., Moore W. H., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins. 3. alpha-Poly(L-alanine). Macromolecules. 1977 Sep-Oct;10(5):1065–1074. doi: 10.1021/ma60059a034. [DOI] [PubMed] [Google Scholar]
- Shriver J., Mateescu G., Fager R., Toricha D., Abrahamson E. W. Unprotonated chromophore-protein bond in visual pigments from 13C-NMR spectra. Nature. 1977 Nov 17;270(5634):271–274. doi: 10.1038/270271a0. [DOI] [PubMed] [Google Scholar]
- Sulkes M., Lewis A., Marcus M. A. Resonance Raman spectroscopy of squid and bovine visual pigments: the primary photochemistry in visual transduction. Biochemistry. 1978 Oct 31;17(22):4712–4722. doi: 10.1021/bi00615a018. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- van der Meer K., Mulder J. J., Lugtenburg J. A new facet in rhodopsin photochemistry. Photochem Photobiol. 1976 Oct;24(4):363–367. doi: 10.1111/j.1751-1097.1976.tb06837.x. [DOI] [PubMed] [Google Scholar]


