Abstract
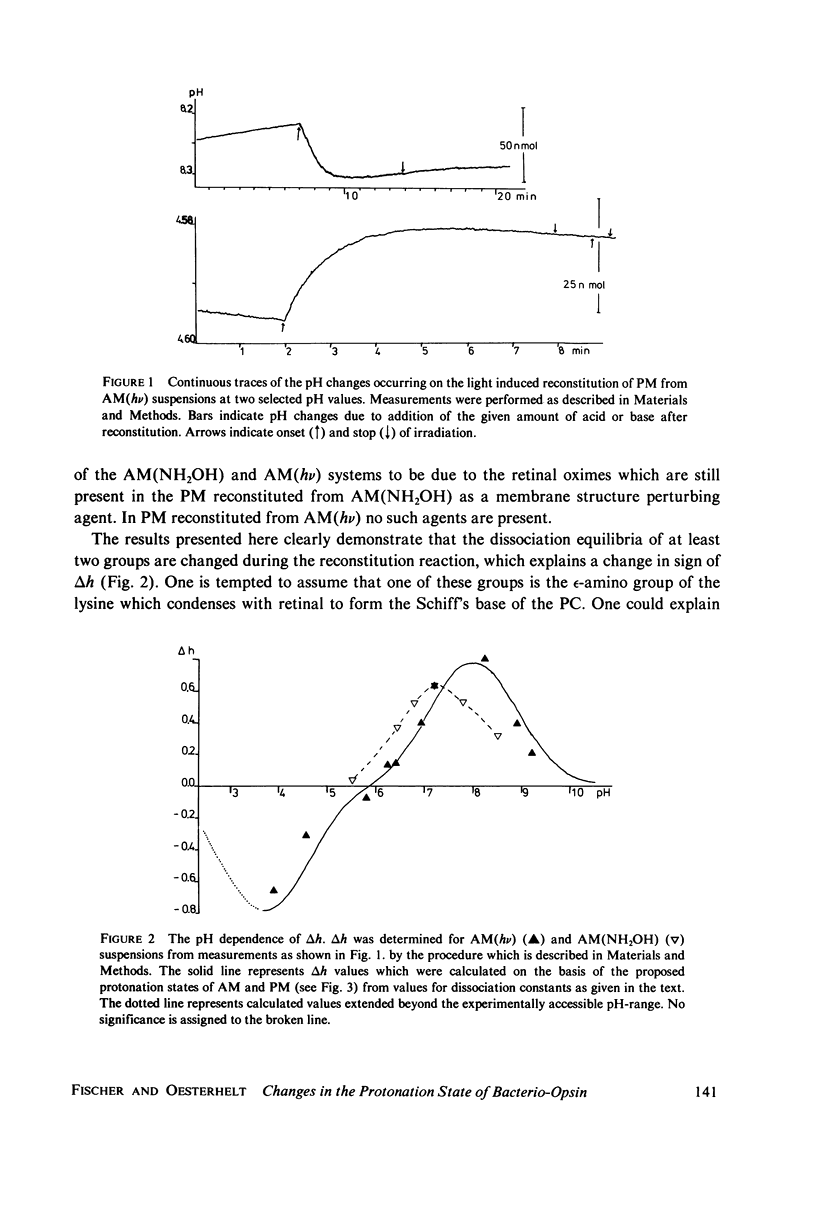
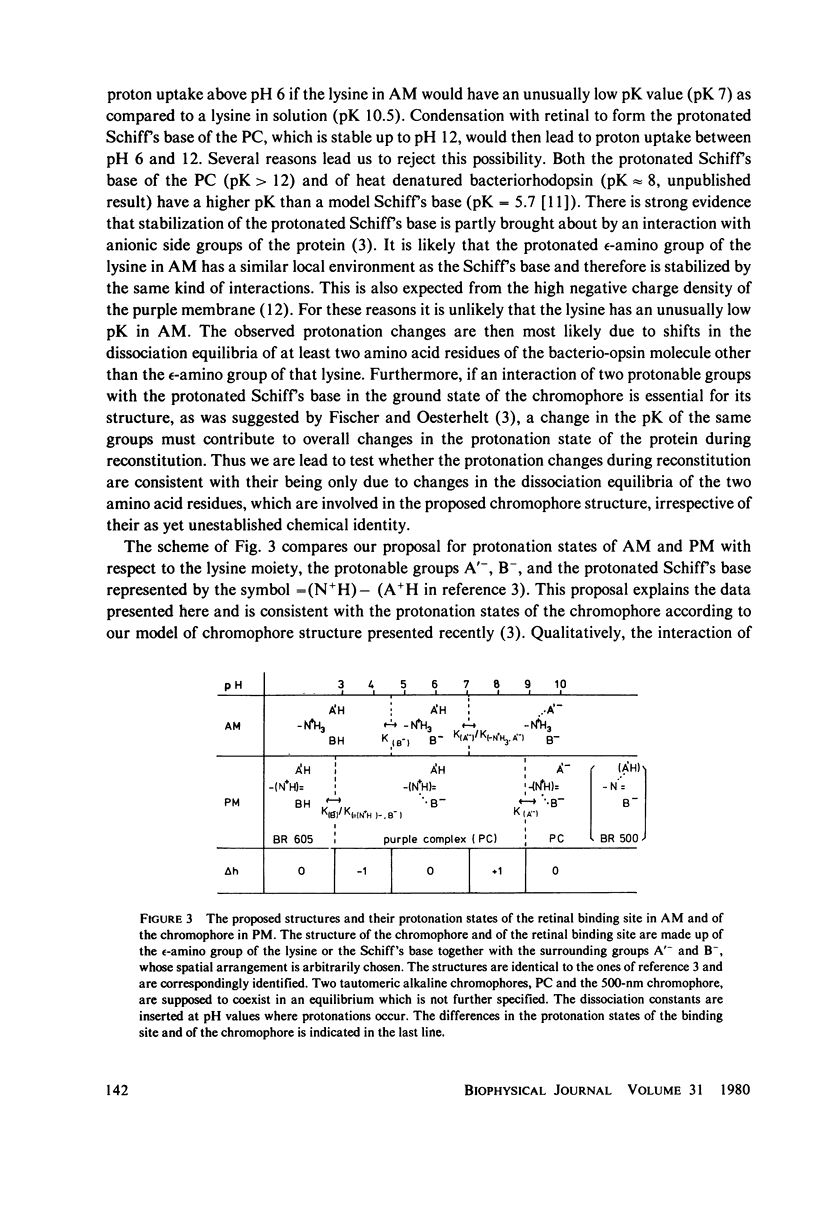
Protonation changes of the protein occur during the reconstitution of bacteriorhodopsin from bacterio-opsin and all-trans retinal in the purple membrane of Halobacterium halobium. The protonation changes are conveniently determined from measures of the pH changes after photoisomerisation of 9-cis retinal in apomembrane preparations, which induces the reconstitution. In addition, to the omega-amino group of the lysine which is involved in the condensation of retinal and bacterio-opsin, the dissociation equilibria of at least two other amino acid residues are changed during the reconstitution. The results are consistent with a proposed model of chromophore structure in which an interaction of the Schiff's base occurs with two protonable amino acid residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz P. E., Mohler J. H., Navangul H. V. Anion-induced wavelength regulation of absorption maxima of Schiff bases of retinal. Biochemistry. 1972 Feb 29;11(5):848–855. doi: 10.1021/bi00755a026. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. A., PITT G. A. Studies on rhodopsin. IX. pH and the hydrolysis of indicator yellow. Biochem J. 1955 Jan;59(1):128–134. doi: 10.1042/bj0590128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer D. C., Oesterhelt D., Zingsheim H. P. The two faces of the purple membrane. II. Differences in surface charge properties revealed by ferritin binding. J Mol Biol. 1978 Oct 25;125(2):123–135. doi: 10.1016/0022-2836(78)90341-8. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L., Gruber H. Light-dependent reaction of bacteriorhodopsin with hydroxylamine in cell suspensions of Halobacterium halobium: demonstration of an apo-membrane. FEBS Lett. 1974 Aug 30;44(3):257–261. doi: 10.1016/0014-5793(74)81152-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]


