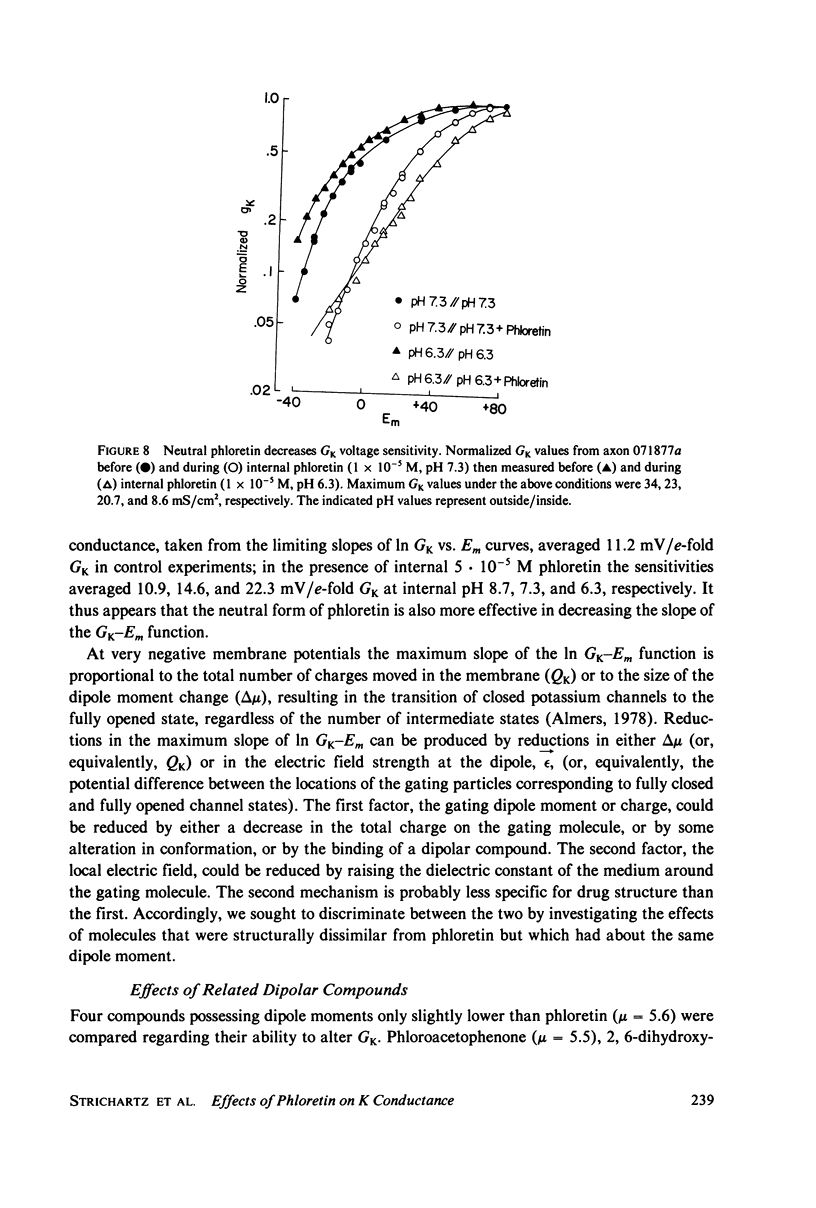
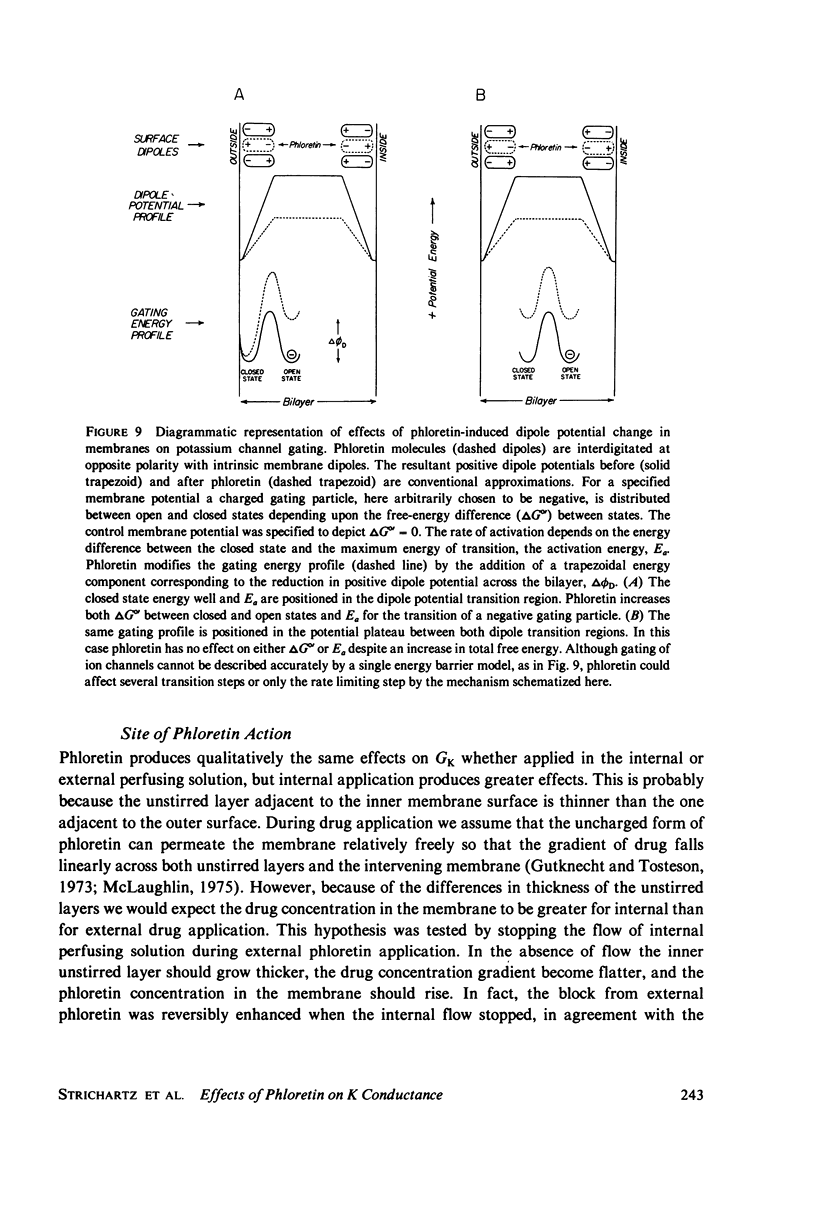
Abstract
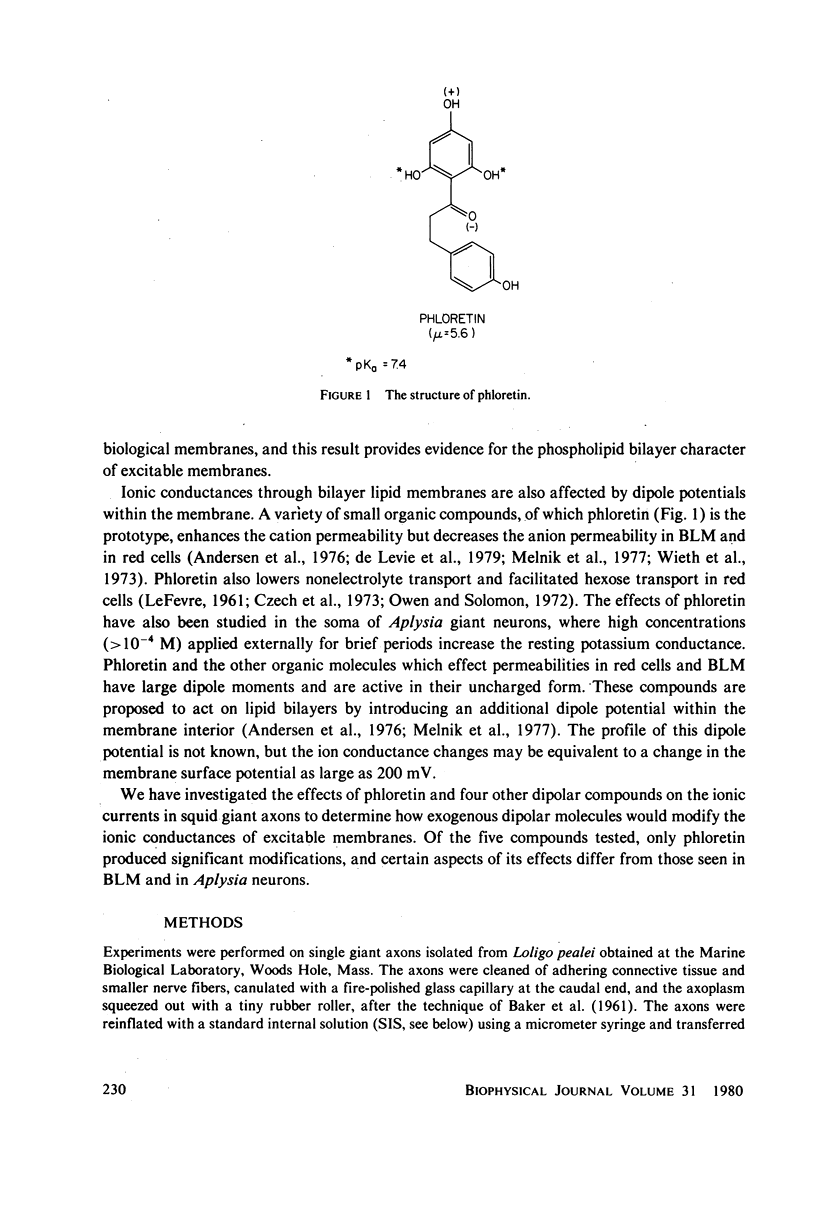
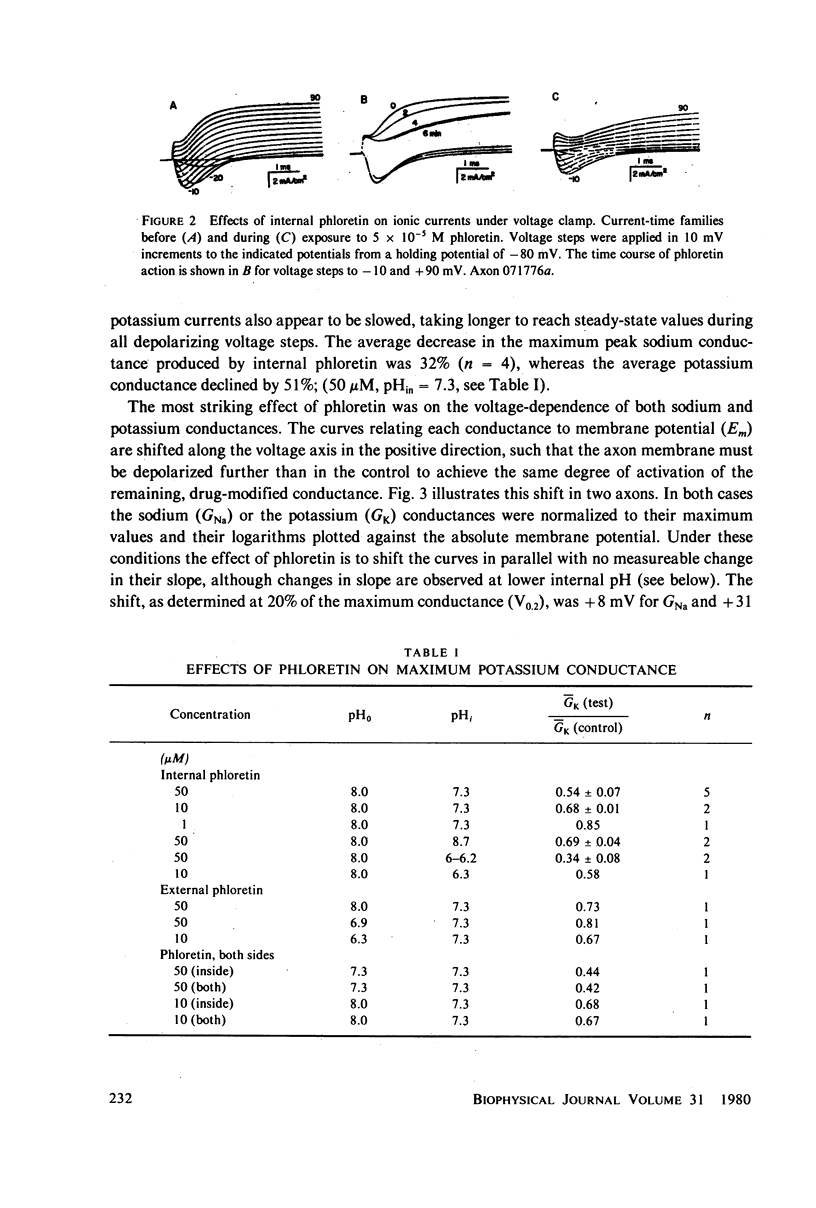
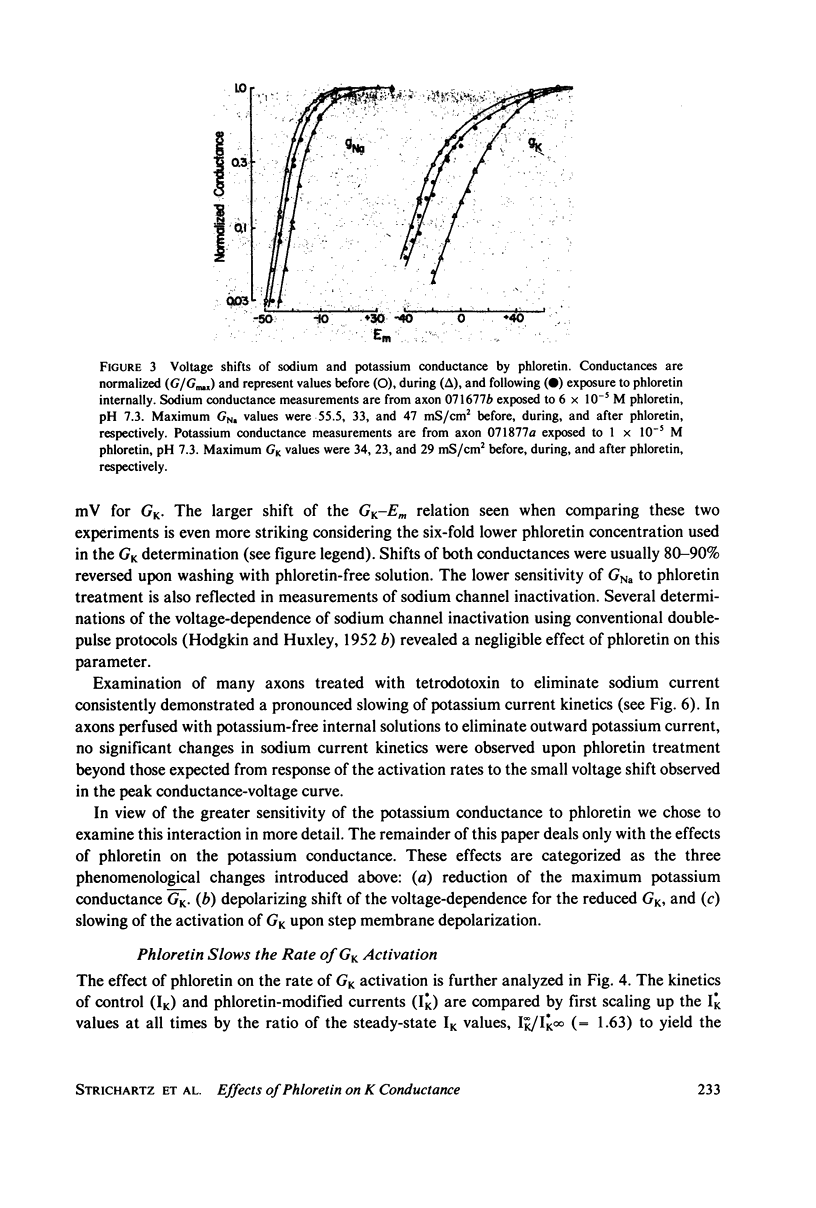
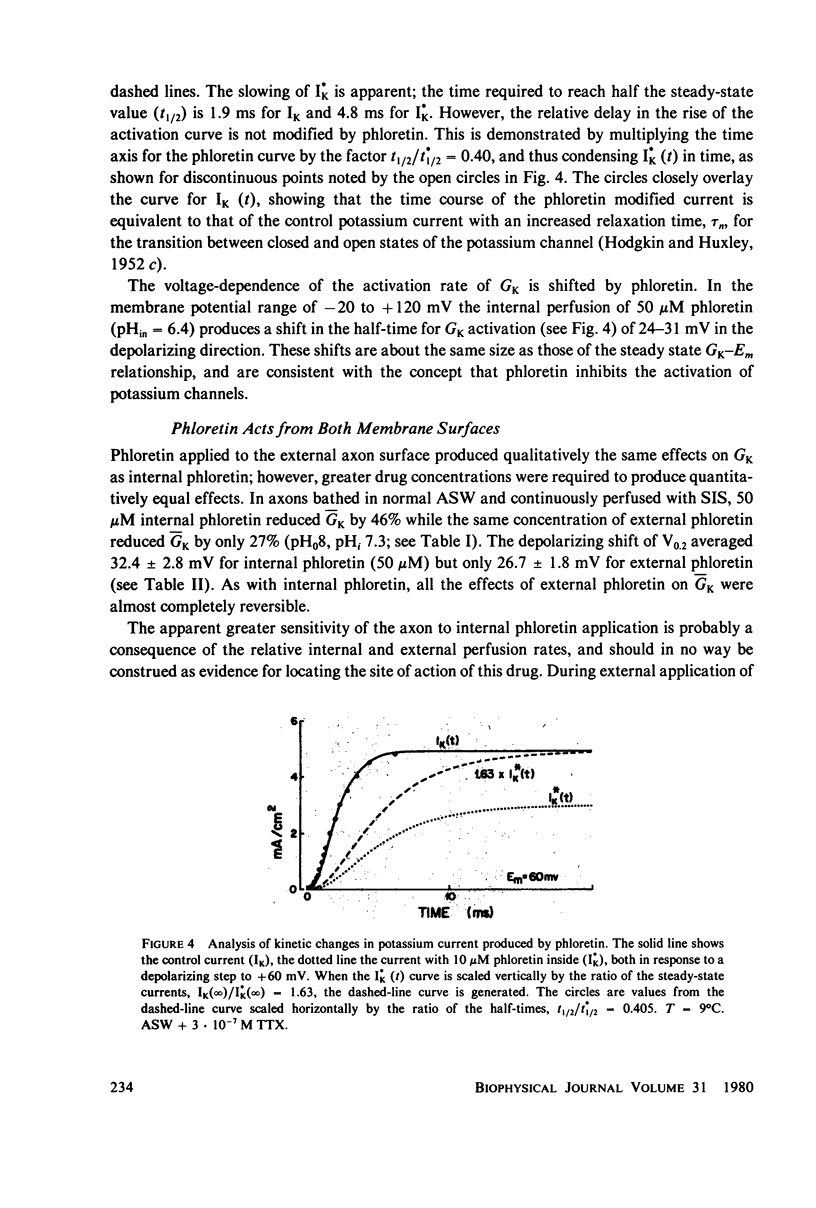
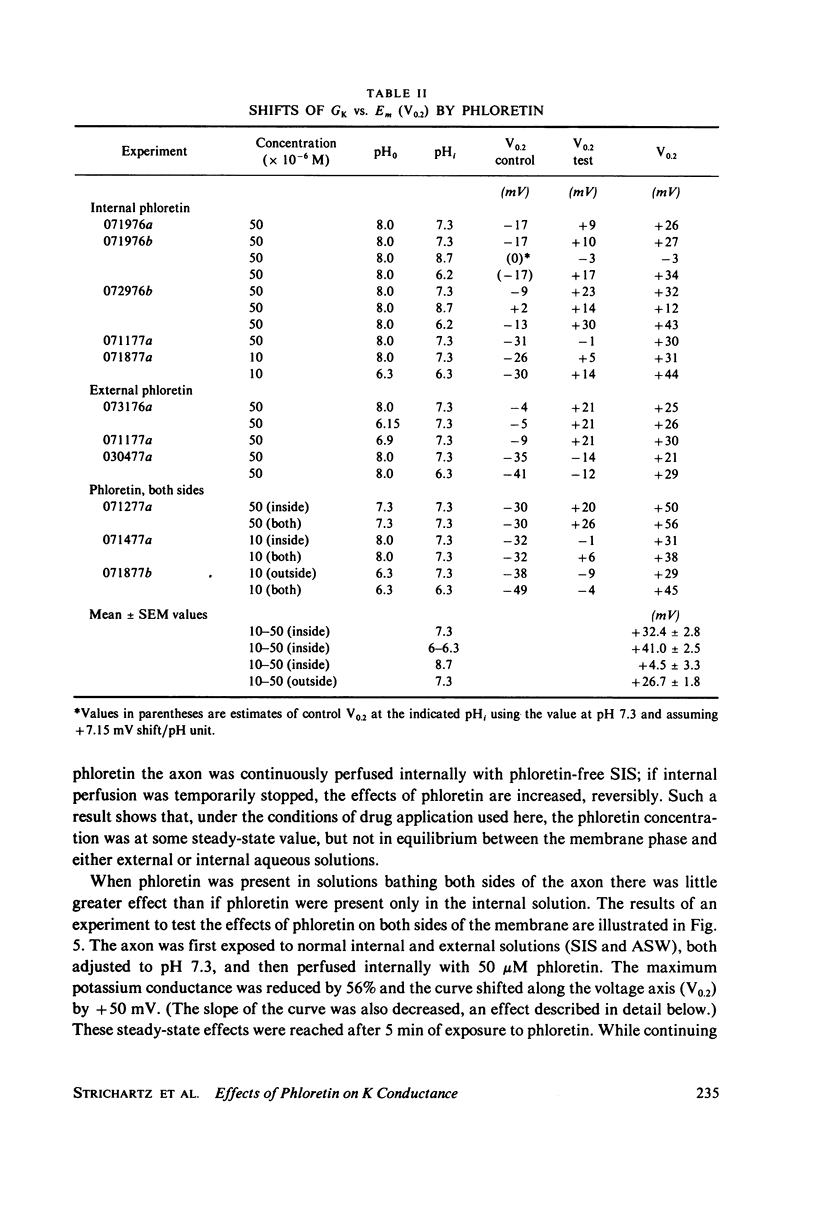
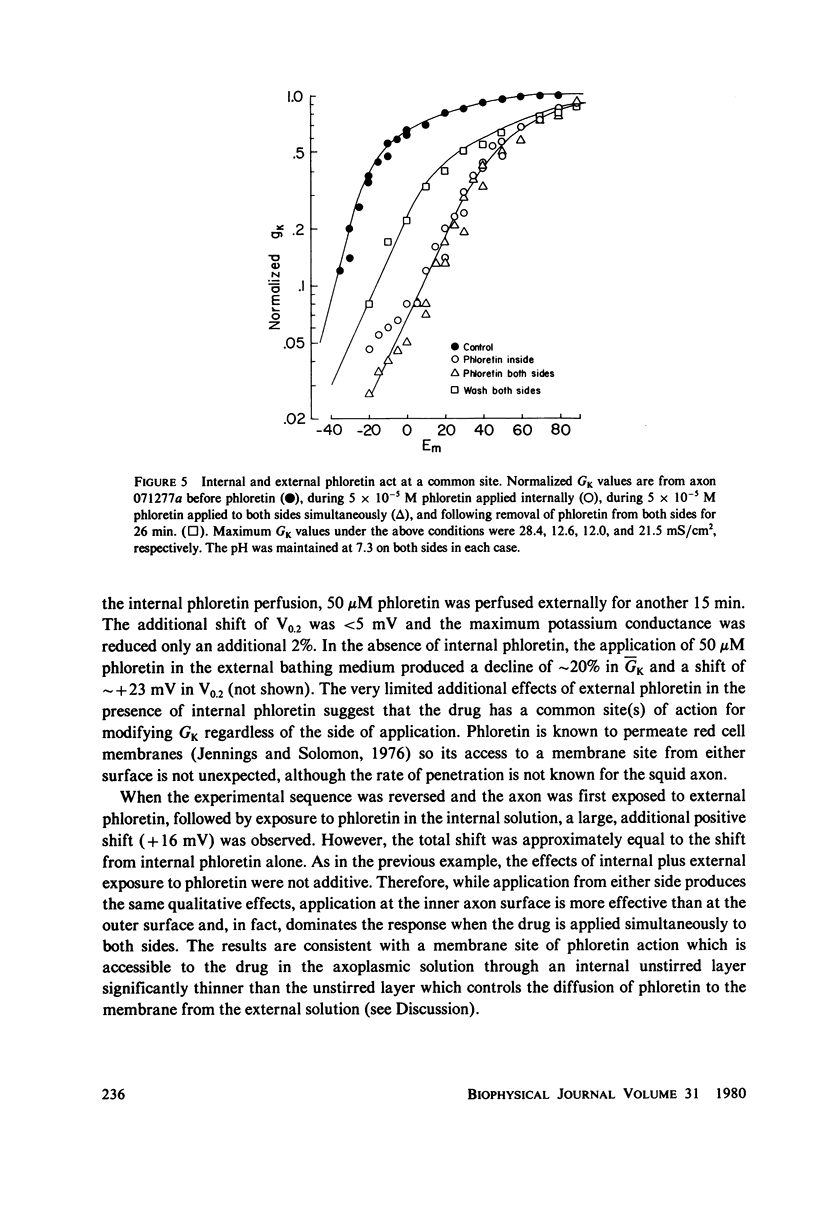
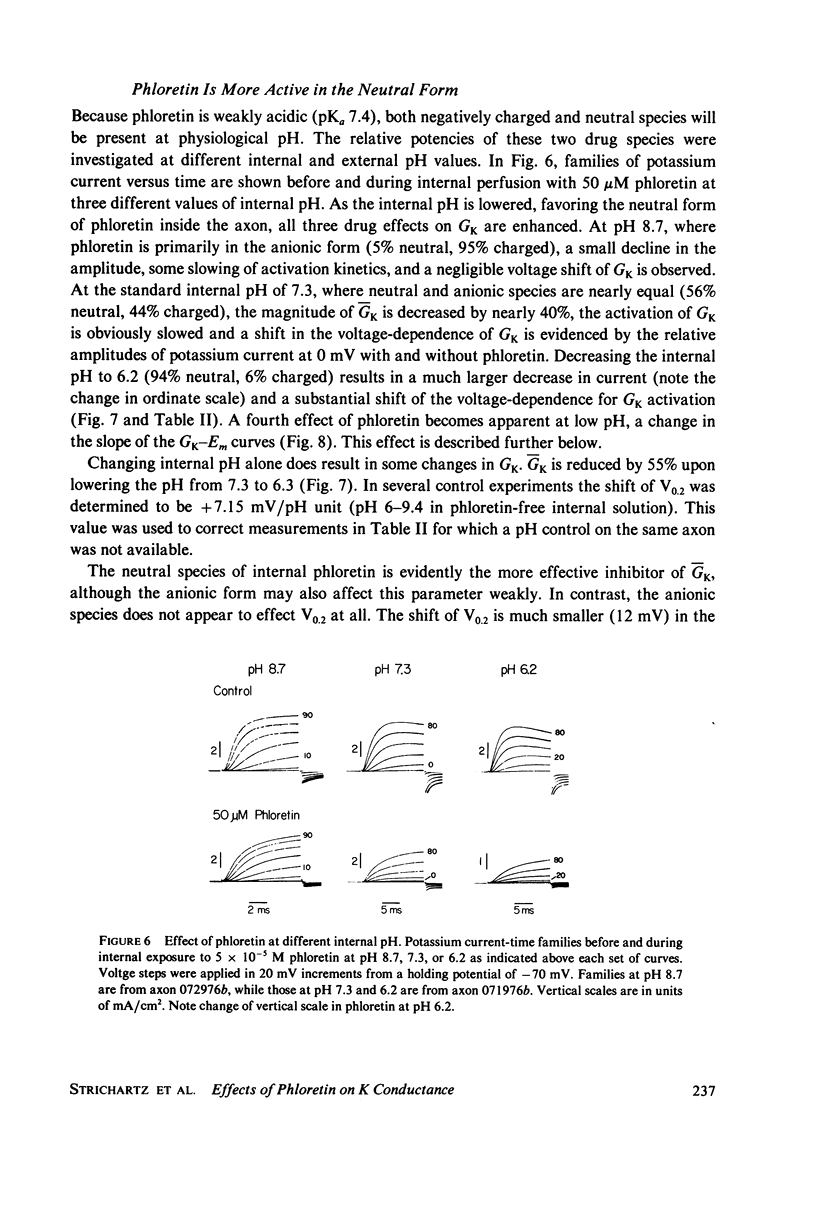
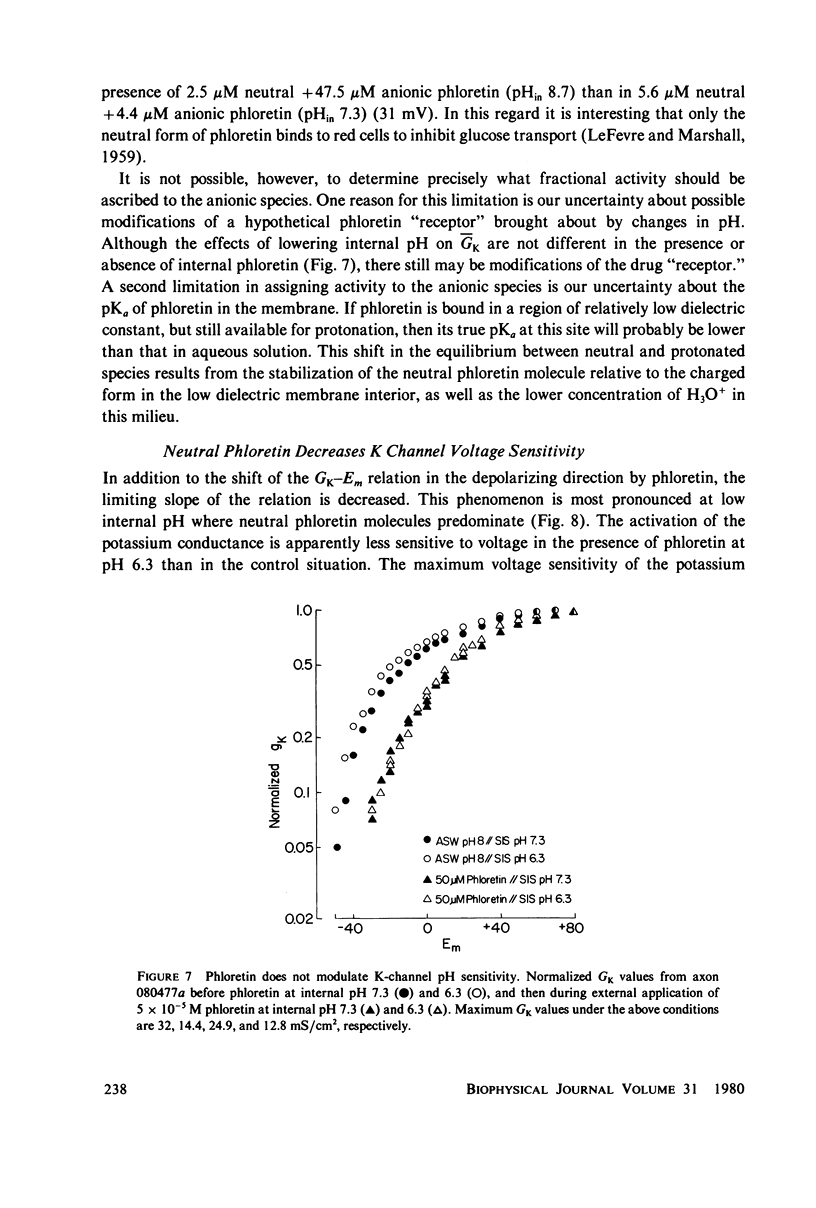
The effects of phloretin on membrane ionic conductances have been studied in the giant axon of the squid, Loligo pealei. Phloretin reversibly suppresses the potassium and sodium conductances and modifies their dependence on membrane potential (Em). Its effects on the potassium conductance (GK) are much greater than on the sodium conductance; no effects on sodium inactivation are observed. Internal perfusion of phloretin produces both greater shifts in GK(Em) and greater reductions maximum GK than does external perfusion; the effect of simultaneous internal and external perfusion is little greater than that of internal perfusion alone. Lowering the internal pH, which favors the presence of the neutral species of weakly acidic phloretin (pKa 7.4), potentiates the actions of internally perfused phloretin. Other organic cations with dipole moments similar to phloretin's have little effect on either potassium or sodium conductances in squid axons. These results can be explained by either of two mechanisms; on postulates a phloretin "receptor" near the voltage sensor component of the potassium channel which is accessible to drug molecules applied at either the outer or inner membrane surface and is much more sensitive to the neutral than the negatively charged form of the drug. The other mechanism proposes that neutral phloretin molecules are dispersed in an ordered array in the membrane interior, producing a diffuse dipole field which modifies potassium channel gating. Different experimental results support these two mechanisms, and neither hypothesis can be disproven.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Currents associated with the ionic gating structures in nerve membrane. Ann N Y Acad Sci. 1975 Dec 30;264:265–277. doi: 10.1111/j.1749-6632.1975.tb31488.x. [DOI] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lynn D. G., Lynn W. S. Cytochalasin B-sensitive 2-deoxy-D-glucose transport in adipose cell ghosts. J Biol Chem. 1973 May 25;248(10):3636–3641. [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969 Mar;9(3):447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Solomon A. K. Interaction between phloretin and the red blood cell membrane. J Gen Physiol. 1976 Apr;67(4):381–397. doi: 10.1085/jgp.67.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. The atachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem. 1959 Nov;234:3022–3026. [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- Levitan E., Palti Y. Dipole moment, enthalpy, and entropy changes of Hodgkin-Huxley type kinetic units. Biophys J. 1975 Mar;15(3):239–251. doi: 10.1016/S0006-3495(75)85815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik E., Latorre R., Hall J. E., Tosteson D. C. Phloretin-induced changes in ion transport across lipid bilayer membranes. J Gen Physiol. 1977 Feb;69(2):243–257. doi: 10.1085/jgp.69.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. D., Solomon A. K. Control of nonelectrolyte permeability in red cells. Biochim Biophys Acta. 1972 Dec 1;290(1):414–418. doi: 10.1016/0005-2736(72)90087-9. [DOI] [PubMed] [Google Scholar]
- Owen J. D. The effect of phloretin on the potassium conductance in Aplysia giant neurons. J Membr Biol. 1974;16(1):65–78. doi: 10.1007/BF01872407. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. K+ conductance modified by a titratable group accessible to protons from the intracellular side of the squid axon membrane. Biophys J. 1979 May;26(2):319–324. doi: 10.1016/S0006-3495(79)85251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Narahashi T. Mechanism of action of propranolol on squid axon membranes. J Pharmacol Exp Ther. 1973 Jan;184(1):155–162. [PubMed] [Google Scholar]
- de Levie R., Rangarajan S. K., Seelig P. F., Andersen O. S. On the adsorption of phloretin onto a black lipid membrane. Biophys J. 1979 Feb;25(2 Pt 1):295–300. doi: 10.1016/s0006-3495(79)85292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


