Abstract
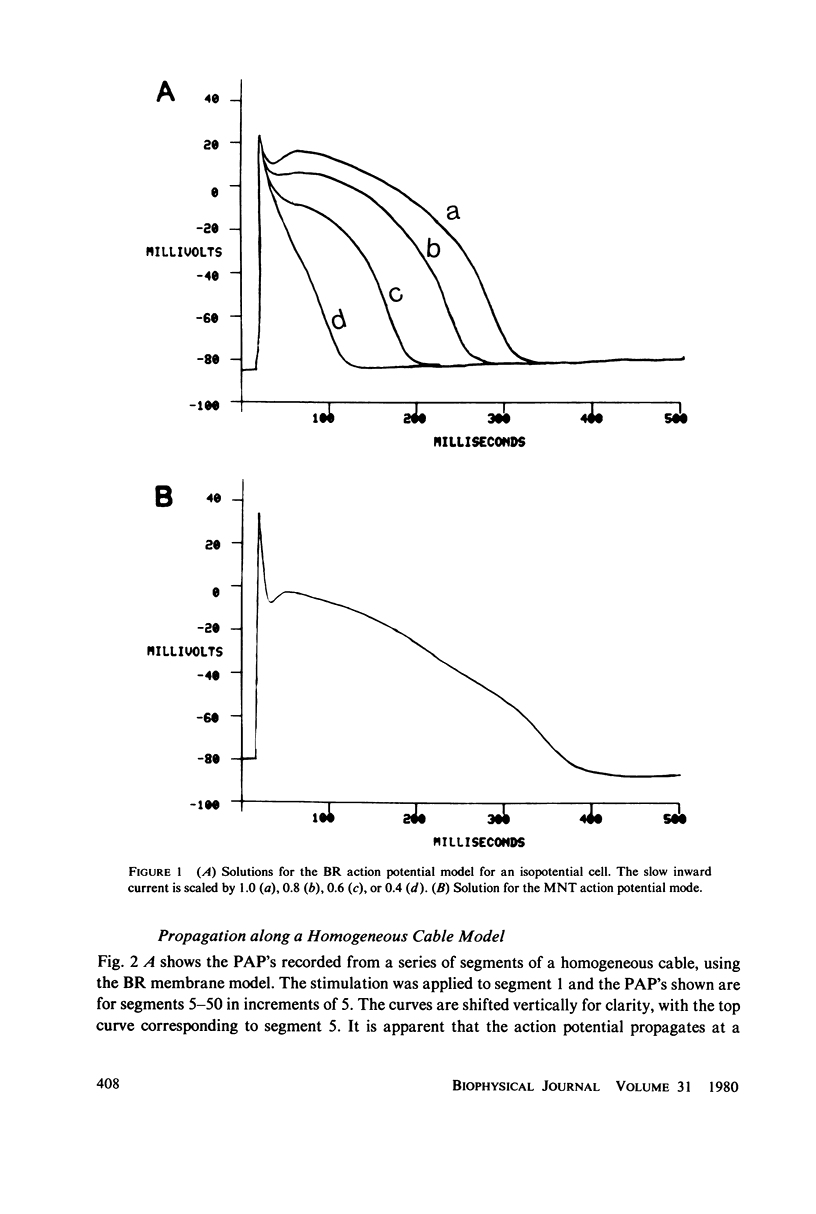
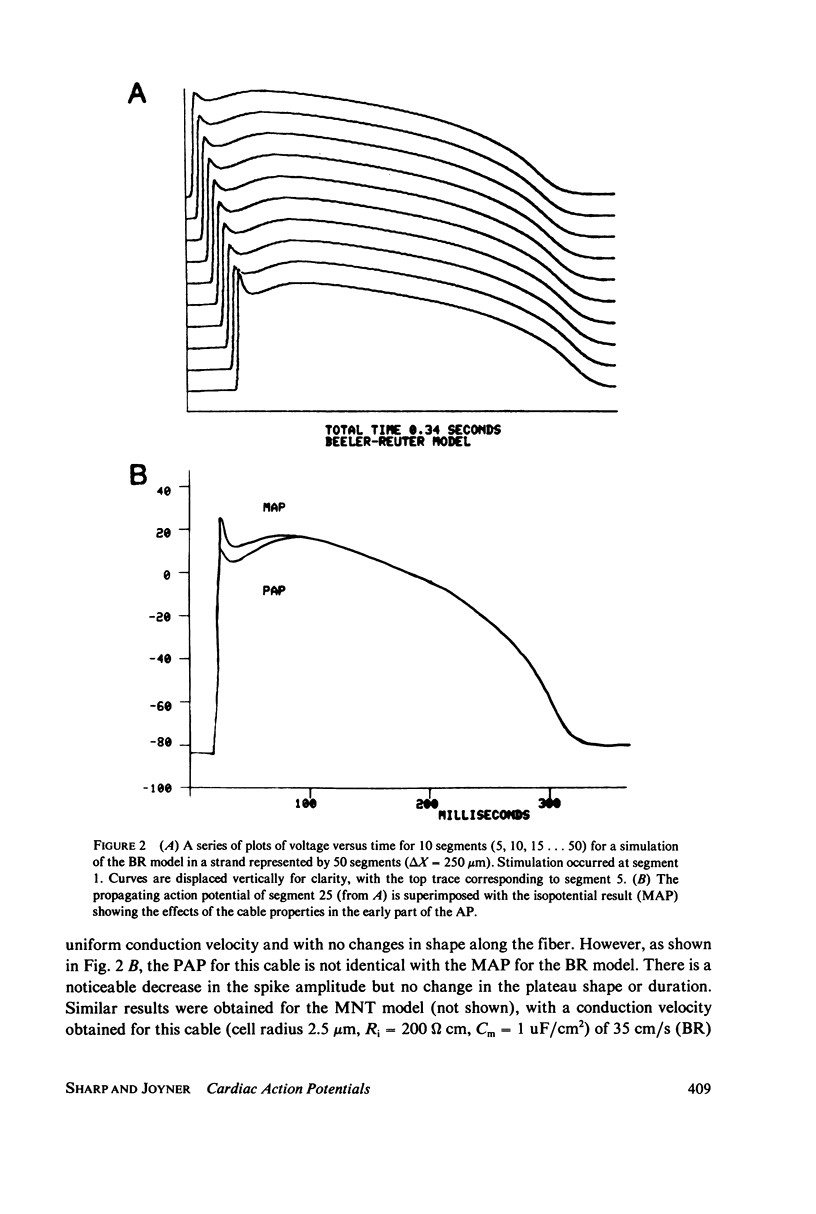
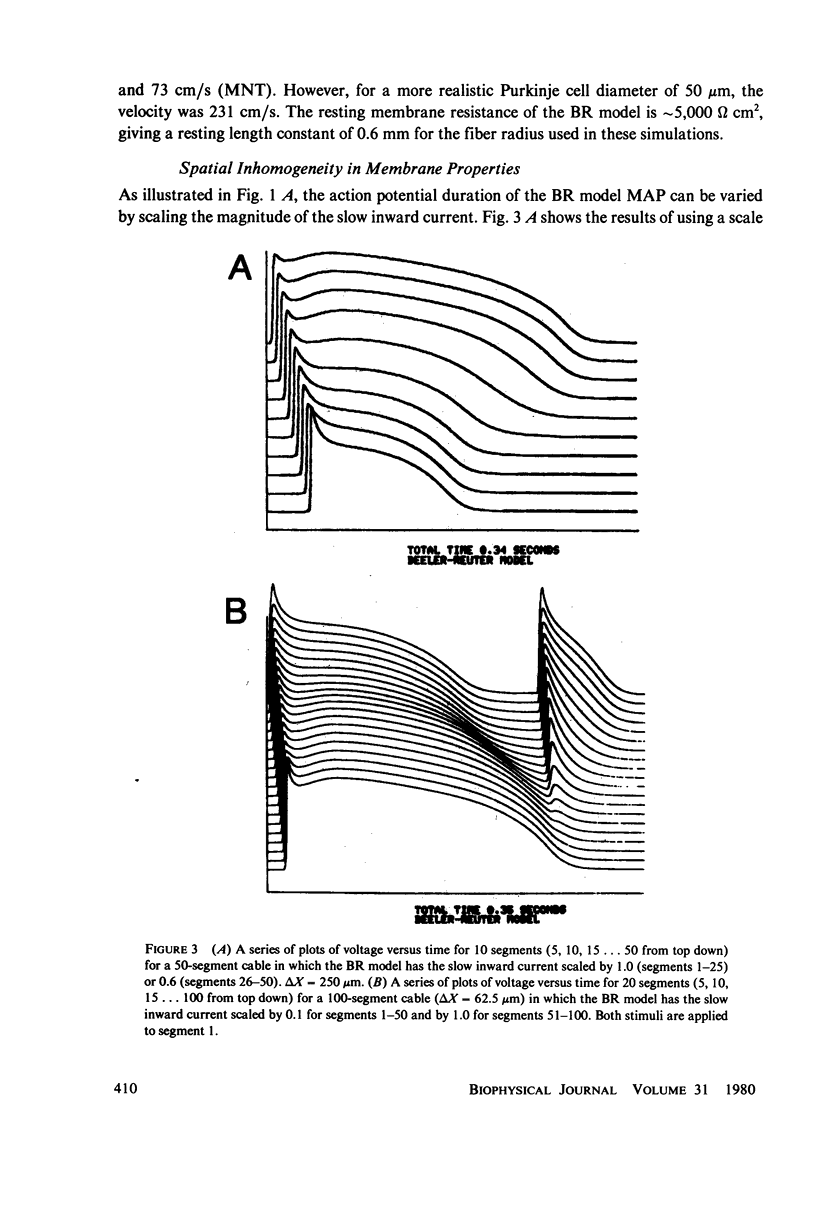
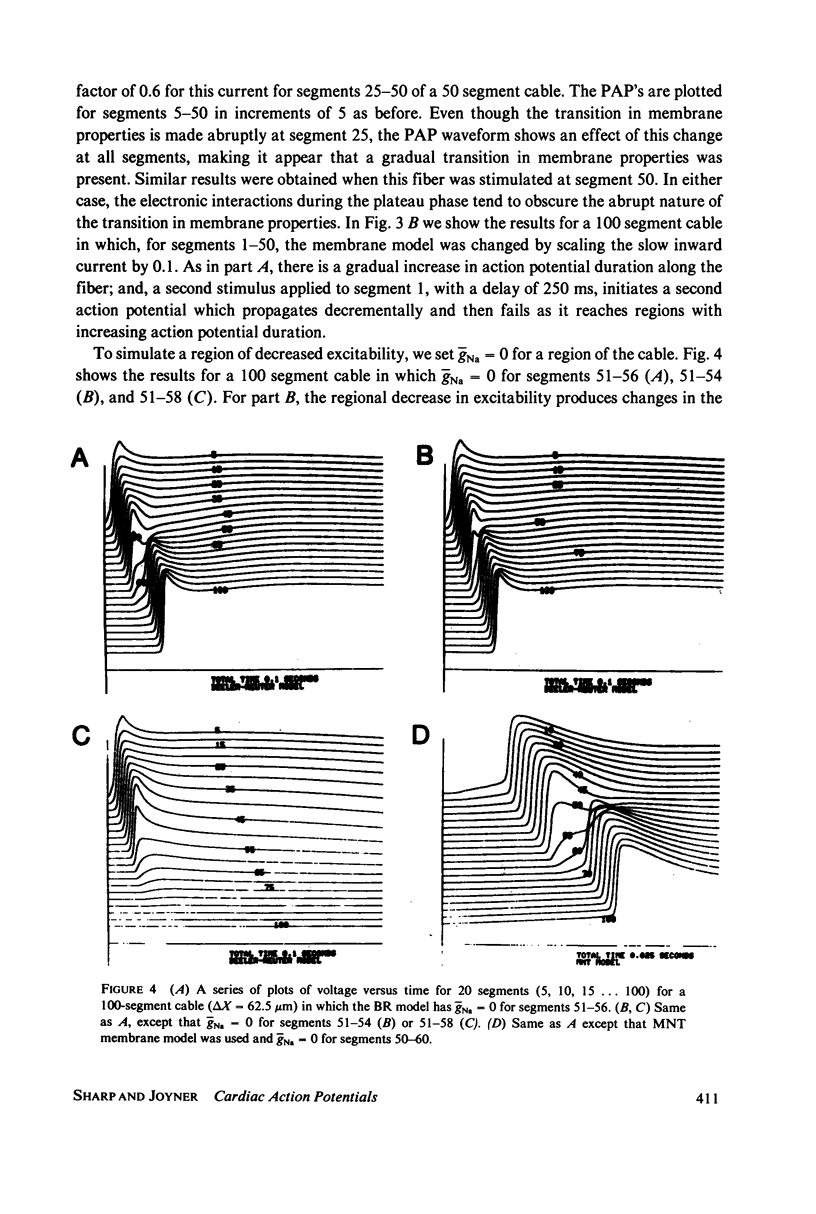
We have used numerical methods for solving cable equations, combined with previously published mathematical models for the membrane properties of ventricular and Purkinje cells, to simulate the propagation of cardiac action potentials along a unidimensional strand. Two types of inhomogeneities have been simulated and the results compared with experimentally observed disturbances in cardiac action potential propagation. Changes in the membrane model for regions of the strand were introduced to simulate regions of decreased excitability. Regional changes in the intercellular coupling were also studied. The results illustrate and help to explain the disturbances in propagation which have been reported to occur at regions of decreased excitability, regions with changing action potential duration, or regions with changing intercellular coupling. The propagational disturbances seen at all of these regions are discussed in terms of the changing electrical load imposed upon the propagating impulse.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. J., Greenspan K., Bandura J. P., Fisch C. Asynchrony of conduction within the canine specialized Purkinje fiber system. Circ Res. 1970 Nov;27(5):691–703. doi: 10.1161/01.res.27.5.691. [DOI] [PubMed] [Google Scholar]
- Attwell D., Cohen I. The voltage clamp of multicellular preparations. Prog Biophys Mol Biol. 1977;31(3):201–245. doi: 10.1016/0079-6107(78)90009-3. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill M. H., Waxman S. G., Moore J. W., Joyner R. W. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry. 1977 Aug;40(8):769–774. doi: 10.1136/jnnp.40.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Vereecke J. Adrenaline and the plateau phase of the cardiac action potential. Importance of Ca++, Na+ and K+ conductance. Pflugers Arch. 1969;313(4):300–315. doi: 10.1007/BF00593955. [DOI] [PubMed] [Google Scholar]
- Challice C. E. Functional morphology of the specialized tissues of the heart. (With plates 1-3). Methods Achiev Exp Pathol. 1971;5:121–172. [PubMed] [Google Scholar]
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley J. W., Dodge F. A., Jr Digital computer solutions for excitation and propagation of the nerve impulse. Biophys J. 1966 Sep;6(5):583–599. doi: 10.1016/S0006-3495(66)86679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranefield P. F., Klein H. O., Hoffman B. F. Conduction of the cardiac impulse. 1. Delay, block, and one-way block in depressed Purkinje fibers. Circ Res. 1971 Feb;28(2):199–219. doi: 10.1161/01.res.28.2.199. [DOI] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Influence of the sodium pump on intercellular communication in heart fibres: effect of intracellular injection of sodium ion on electrical coupling. J Physiol. 1976 Dec;263(2):171–197. doi: 10.1113/jphysiol.1976.sp011627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente D., Sasyniuk B., Moe G. K. Conduction through a narrow isthmus in isolated canine atrial tissue. A model of the W-P-W syndrome. Circulation. 1971 Nov;44(5):803–809. doi: 10.1161/01.cir.44.5.803. [DOI] [PubMed] [Google Scholar]
- Goldstein S. S., Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J. 1974 Oct;14(10):731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. The effect of tetraethylammonium chloride on the muscle membrane examined with an intracellular microelectrode. J Physiol. 1955 Sep 28;129(3):513–527. doi: 10.1113/jphysiol.1955.sp005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN B. F., CRANEFIELD P. F. THE PHYSIOLOGICAL BASIS OF CARDIAC ARRHYTHMIAS. Am J Med. 1964 Nov;37:670–684. doi: 10.1016/0002-9343(64)90017-8. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Ramón F., Morre J. W. Simulation of action potential propagation in an inhomogeneous sheet of coupled excitable cells. Circ Res. 1975 May;36(5):654–661. doi: 10.1161/01.res.36.5.654. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Westerfield M., Moore J. W., Stockbridge N. A numerical method to model excitable cells. Biophys J. 1978 May;22(2):155–170. doi: 10.1016/S0006-3495(78)85481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G., Janse M. J., van Capelle F. J., Durrer D. Mechanism and time course of S-T and T-Q segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circ Res. 1978 May;42(5):603–613. doi: 10.1161/01.res.42.5.603. [DOI] [PubMed] [Google Scholar]
- Kupersmith J., Shiang H., Litwak R. S., Herman M. V. Electrophysiological and antiarrhythmic effects of propranolol in canine acute myocardial ischemia. Circ Res. 1976 Apr;38(4):302–307. doi: 10.1161/01.res.38.4.302. [DOI] [PubMed] [Google Scholar]
- Lazzara R., El-Sherif N., Scherlag B. J. Disorders of cellular electrophysiology produced by ischemia of the canine His bundle. Circ Res. 1975 Mar;36(3):444–454. doi: 10.1161/01.res.36.3.444. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kootsey J. M., Johnson E. A., Sawanobori T. Low conduction in cardiac muscle. Biophysical model. Biophys J. 1973 Jan;13(1):37–55. doi: 10.1016/s0006-3495(73)85968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Sawanobori T., Kootsey J. M., Johnson E. A. A synthetic strand of cardiac muscle: its passive electrical properties. J Gen Physiol. 1975 Apr;65(4):527–550. doi: 10.1085/jgp.65.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOE G. K., PRESTON J. B., BURLINGTON H. Physiologic evidence for a dual A-V transmission system. Circ Res. 1956 Jul;4(4):357–375. doi: 10.1161/01.res.4.4.357. [DOI] [PubMed] [Google Scholar]
- MOE G. K., RHEINBOLDT W. C., ABILDSKOV J. A. A COMPUTER MODEL OF ATRIAL FIBRILLATION. Am Heart J. 1964 Feb;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- MOORE E. N., MORSE H. T., PRICE H. L. CARDIAC ARRHYTHMIAS PRODUCED BY CATECHOLAMINES IN ANESTHETIZED DOGS. Circ Res. 1964 Jul;15:77–82. doi: 10.1161/01.res.15.1.77. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Alanis J., Benitez D. Transitional cardiac cells of the conductive system of the dog heart. Distinguishing morphological and electrophysiological features. J Cell Biol. 1970 Oct;47(1):1–17. doi: 10.1083/jcb.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez C., Mueller W. J., Merideth J., Moe G. K. Interaction of transmembrane potentials in canine Purkinje fibers and at Purkinje fiber-muscle junctions. Circ Res. 1969 Mar;24(3):361–372. doi: 10.1161/01.res.24.3.361. [DOI] [PubMed] [Google Scholar]
- Mendez C., Mueller W. J., Urguiaga X. Propagation of impulses across the Prukinje fiber-muscle junctions in the dog heart. Circ Res. 1970 Feb;26(2):135–150. doi: 10.1161/01.res.26.2.135. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Joyner R. W., Brill M. H., Waxman S. D., Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978 Feb;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramón F., Joyner R. W. Axon voltage-clamp simulations. I. Methods and tests. Biophys J. 1975 Jan;15(1):11–24. doi: 10.1016/S0006-3495(75)85788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerburg R. J., Stewart J. W., Hoffman B. F. Electrophysiological propertiesf the canine peripheral A-V conducting system. Circ Res. 1970 Mar;26(3):361–378. doi: 10.1161/01.res.26.3.361. [DOI] [PubMed] [Google Scholar]
- Pollack G. H. Intercellular coupling in the atrioventricular node and other tissues of the rabbit heart. J Physiol. 1976 Feb;255(1):275–298. doi: 10.1113/jphysiol.1976.sp011280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBLUETH A. Ventricular echoes. Am J Physiol. 1958 Oct;195(1):53–60. doi: 10.1152/ajplegacy.1958.195.1.53. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- Sasyniuk B. I., Mendez C. A mechanism for reentry in canine ventricualar tissue. Circ Res. 1971 Jan;28(1):3–15. doi: 10.1161/01.res.28.1.3. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry. 1974 Feb;37(2):152–161. doi: 10.1136/jnnp.37.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Purkinje fibers of the heart examined with the peroxidase reaction. J Cell Biol. 1968 May;37(2):570–574. doi: 10.1083/jcb.37.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry F. H., Wennemark J. R., Brody D. A. Numerical simulation of conduction delay in blocked Purkinje tissue. Circ Res. 1972 Jul;31(1):53–64. doi: 10.1161/01.res.31.1.53. [DOI] [PubMed] [Google Scholar]
- WALLACE A. G., DAGGETT W. M. RE-EXCITATION OF THE ATRIUM. "THE ECHO PHENOMENON". Am Heart J. 1964 Nov;68:661–666. doi: 10.1016/0002-8703(64)90274-1. [DOI] [PubMed] [Google Scholar]
- Wennemark J. R., Ruesta V. J., Brody D. A. Microelectrode study of delayed conduction in the canine right bundle branch. Circ Res. 1968 Dec;23(6):753–769. doi: 10.1161/01.res.23.6.753. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Hoffman B. F., Cranefield P. F. Slow conduction and reentry in the ventricular conducting system. I. Return extrasystole in canine Purkinje fibers. Circ Res. 1972 Jan;30(1):1–10. doi: 10.1161/01.res.30.1.1. [DOI] [PubMed] [Google Scholar]


