Abstract
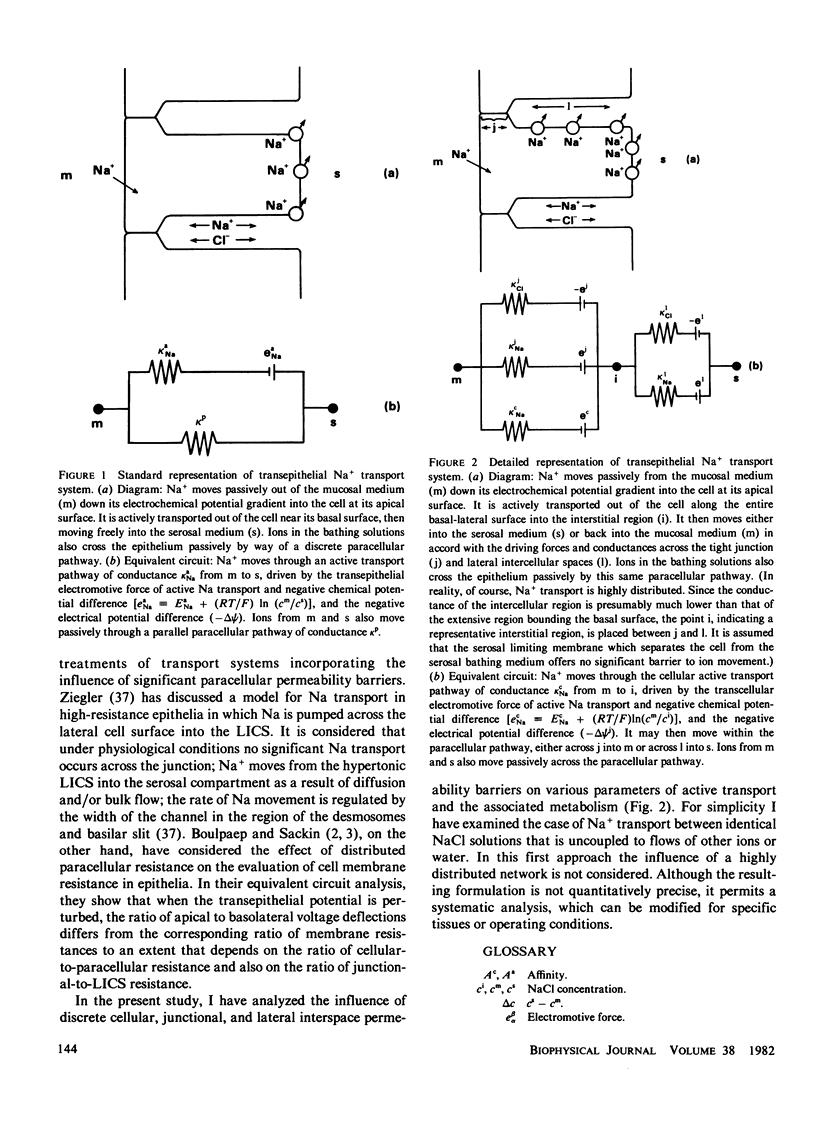
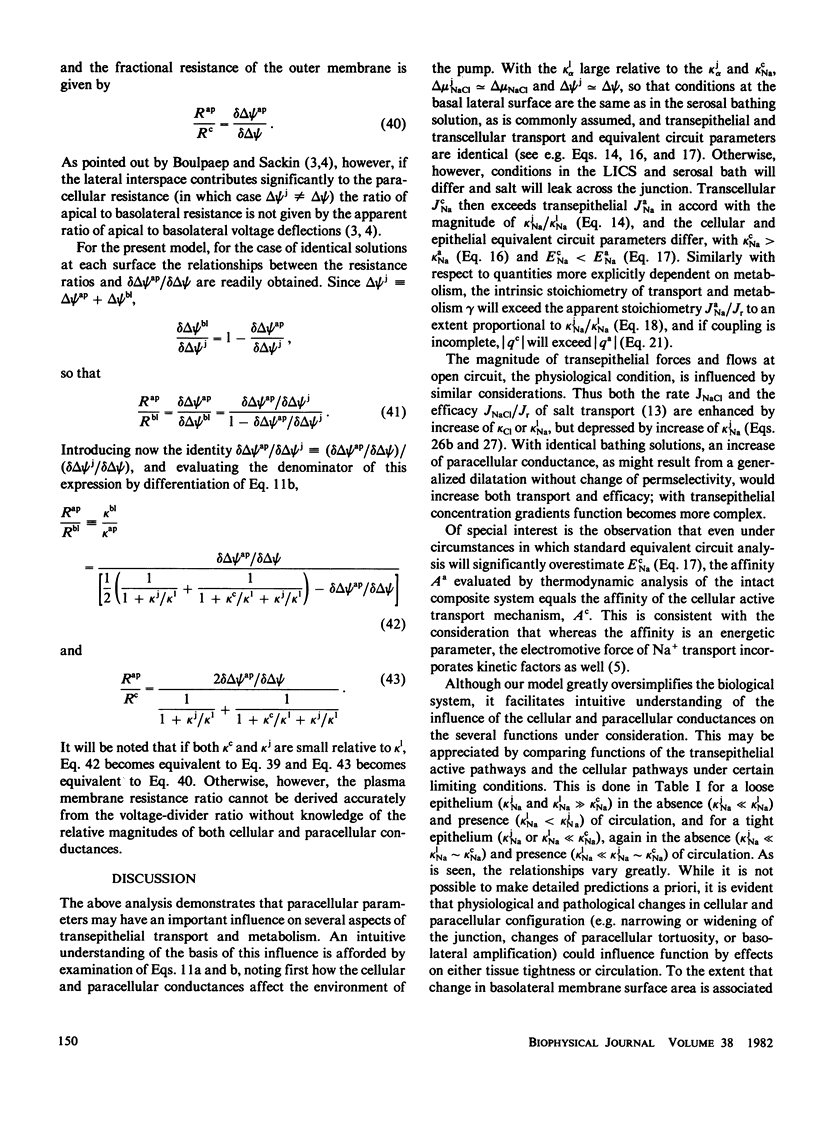
Theoretical analysis of transepithelial active Na transport is often based on equivalent electrical circuits comprising discrete parallel active and passive pathways. Recent findings show, however, that Na+ pumps are distributed over the entire basal lateral surface of epithelial cells. This suggests that Na+ that has been actively transported into paracellular channels may to some extent return to the apical (mucosal) bathing solution, depending on the relative conductances of the pathways via the tight junctions and the lateral intercellular spaces. Such circulation, as well as the relative conductance of cellular and paracellular pathways, may have an important influence on the relationships between parameters of transcellular and transepithelial active transport and metabolism. These relationships were examined by equivalent circuit analysis of active Na transport, Na conductance, the electromotive force of Na transport, the "stoichiometry" of transport, and the degree of coupling of transport to metabolism. Although the model is too crude to permit precise quantification, important qualitative differences are predicted between "loose" and "tight" epithelia in the absence and presence of circulation. In contrast, there is no effect on the free energy of metabolic reaction estimated from a linear thermodynamic formalism. Also of interest are implications concerning the experimental evaluation of passive paracellular conductance following abolition of active transport, and the use of the cellular voltage-divider ratio to estimate the relative conductances of apical and basal lateral plasma membranes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber T. U., Walker T. C., Mullen T. L. Influence of extracellular Cl concentration on Cl transport across isolated skin or Rana pipiens. J Membr Biol. 1980 Aug 21;56(1):81–92. doi: 10.1007/BF01869355. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L., Sackin H. Equivalent electrical circuit analysis and rheogenic pumps in epithelia. Fed Proc. 1979 May;38(6):2030–2036. [PubMed] [Google Scholar]
- CURRAN P. F. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol. 1960 Jul;43:1137–1148. doi: 10.1085/jgp.43.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., DiBona D. R. Pathways for movement of ions and water across toad urinary bladder. III. Physiologic significance of the paracellular pathway. J Membr Biol. 1978 Feb 3;38(4):359–386. doi: 10.1007/BF01870152. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M. Pathways for movement of ions and water across toad urinary bladder. I. Anatomic site of transepithelial shunt pathways. J Membr Biol. 1973;12(2):101–128. doi: 10.1007/BF01869994. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Mills J. W. Distribution of Na+-pump sites in transporting epithelia. Fed Proc. 1979 Feb;38(2):134–143. [PubMed] [Google Scholar]
- DiBona D. R. Passive intercellular pathway in amphibian epithelia. Nat New Biol. 1972 Aug 9;238(84):179–181. doi: 10.1038/newbio238179a0. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig A., Caplan S. R. Energetics of active transport processes. Biophys J. 1968 Dec;8(12):1434–1457. doi: 10.1016/S0006-3495(68)86565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L., Bright J. The paracellular pathway in toad urinary bladder: permselectivity and kinetics of opening. J Membr Biol. 1978 Dec 8;44(1):67–83. doi: 10.1007/BF01940574. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Schultz S. G. Ionic conductances of extracellular shunt pathway in rabbit ileum. Influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol. 1972 Mar;59(3):318–346. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. G. Effect of amiloride on conductance of toad urinary bladder. J Membr Biol. 1980 Jan 31;52(1):61–67. doi: 10.1007/BF01869006. [DOI] [PubMed] [Google Scholar]
- Gupta B. L., Hall T. A., Naftalin R. J. Microprobe measurement of Na, K and Cl concentration profiles in epithelial cells and intercellular spaces of rabbit ileum. Nature. 1978 Mar 2;272(5648):70–73. doi: 10.1038/272070a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. D., Essig A. Effects of 2-deoxy-D-glucose, amiloride, vasopressin, and ouabain on active conductance and ENa in the toad bladder. J Membr Biol. 1976 Aug 26;28(2-3):121–142. doi: 10.1007/BF01869693. [DOI] [PubMed] [Google Scholar]
- Labarca P., Canessa M., Leaf A. Metabolic cost of sodium transport in toad urinary bladder. J Membr Biol. 1977 Apr 22;32(3-4):383–401. doi: 10.1007/BF01905229. [DOI] [PubMed] [Google Scholar]
- Mills J. W., Ernst S. A. Localization of sodium pump sites in frog urinary bladder. Biochim Biophys Acta. 1975 Jan 28;375(2):268–273. doi: 10.1016/0005-2736(75)90194-7. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Effects of luminal hyperosmolality on electrical pathways of Necturas gallbladder. Am J Physiol. 1977 Mar;232(3):C99–108. doi: 10.1152/ajpcell.1977.232.3.C99. [DOI] [PubMed] [Google Scholar]
- Saito T., Lief P. D., Essig A. Conductance of active and passive pathways in the toad bladder. Am J Physiol. 1974 Jun;226(6):1265–1271. doi: 10.1152/ajplegacy.1974.226.6.1265. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Application of equivalent electrical circuit models to study of sodium transport across epithelial tissues. Fed Proc. 1979 May;38(6):2024–2029. [PubMed] [Google Scholar]
- Spring K. R., Hope A. Dimensions of cells and lateral intercellular spaces in living Necturus gallbladder. Fed Proc. 1979 Feb;38(2):128–133. [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Wolff D., Essig A. Protocol-dependence of equivalent circuit parameters of toad urinary bladder. J Membr Biol. 1980 Jun 30;55(1):53–68. doi: 10.1007/BF01926369. [DOI] [PubMed] [Google Scholar]
- Ziegler T. W. A new model for regulation of sodium transport in high resistance epithelia. Med Hypotheses. 1976 May-Jun;2(3):85–96. doi: 10.1016/0306-9877(76)90050-5. [DOI] [PubMed] [Google Scholar]


