Abstract
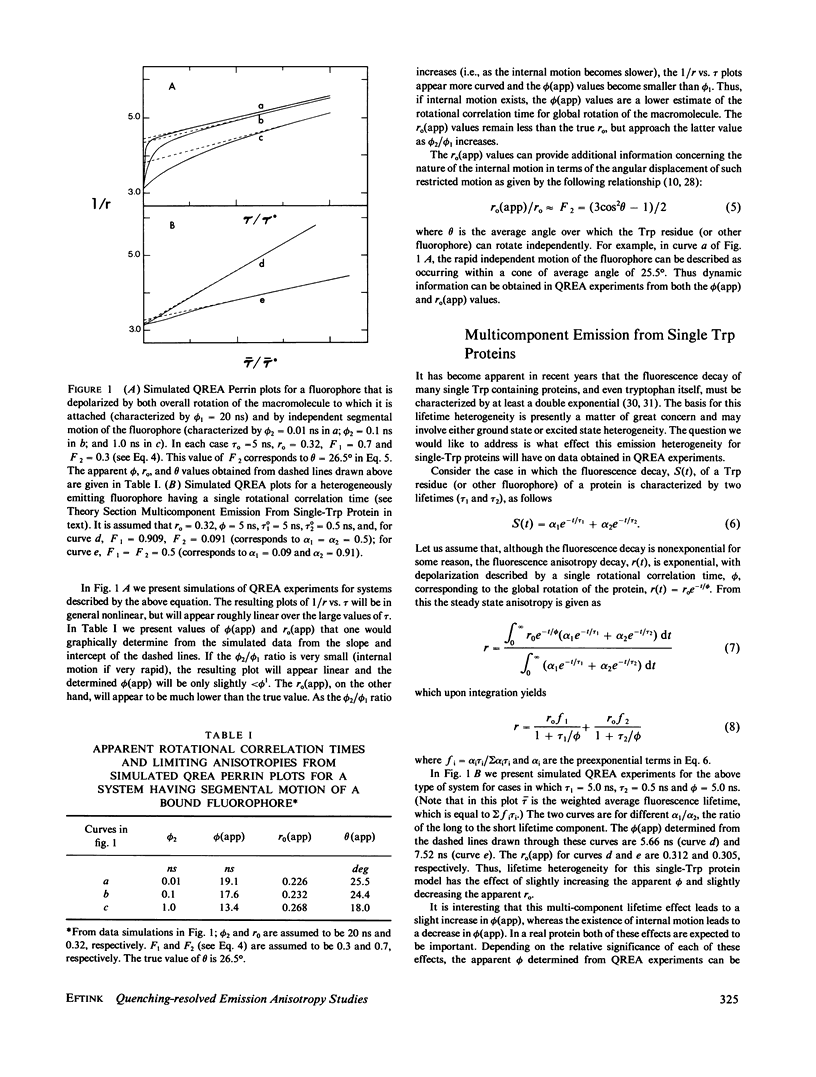
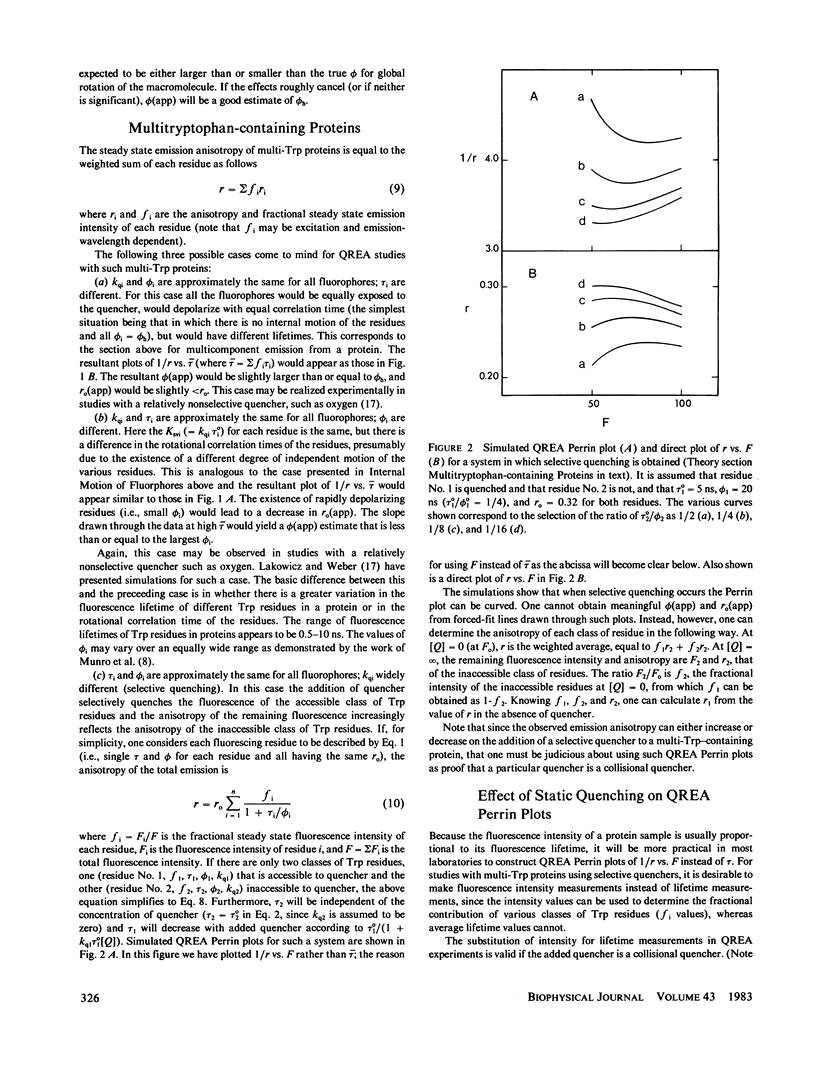
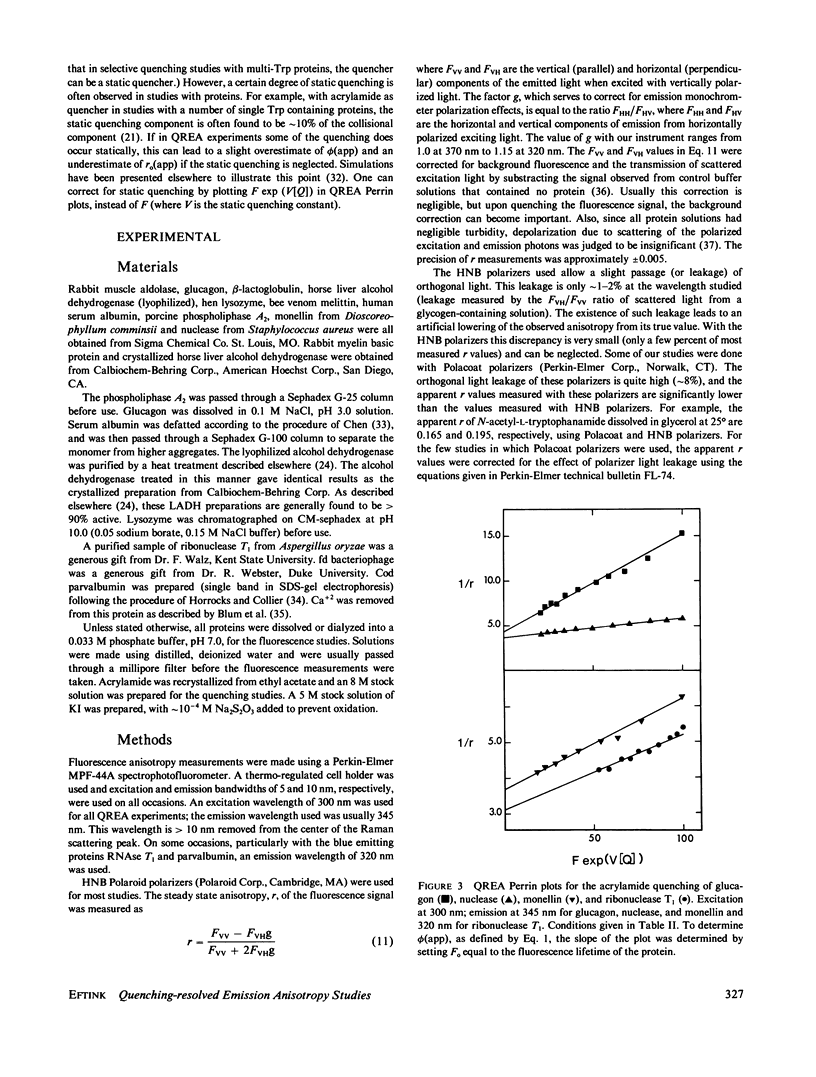
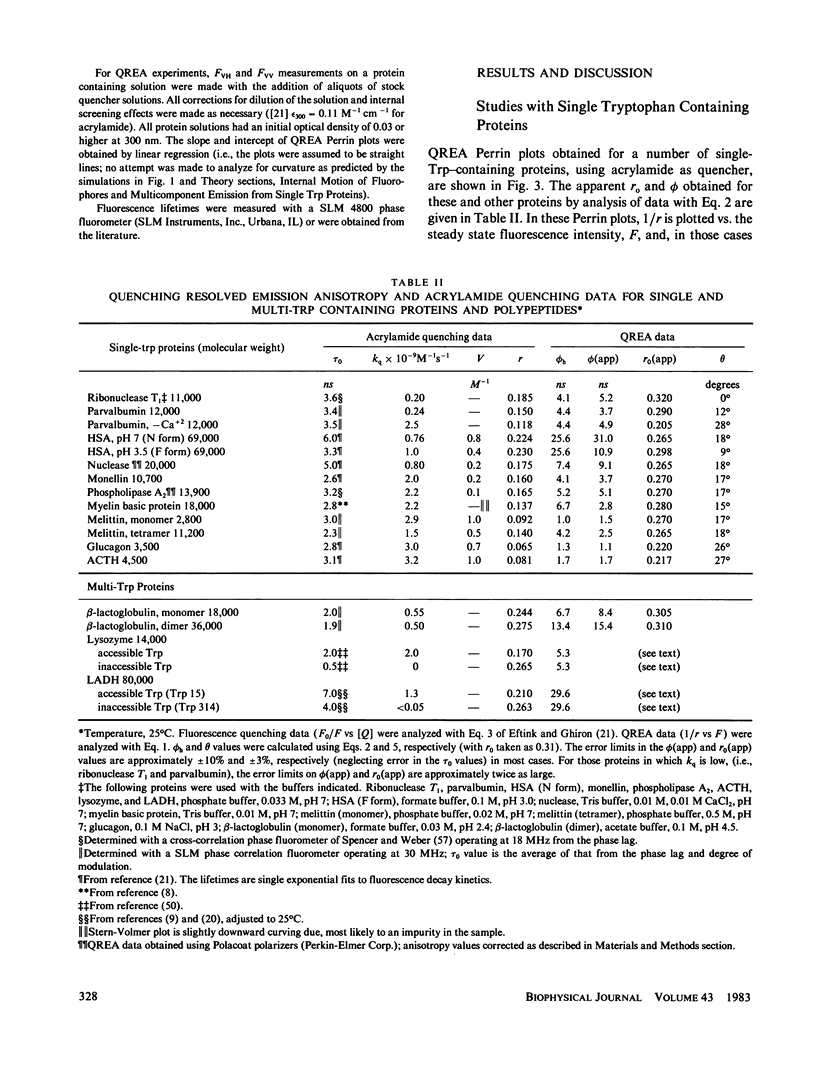
Measurements of the anisotropy of protein fluorescence as a function of an added collisional quencher, such as acrylamide, are used to construct Perrin plots. For single tryptophan containing proteins, such plots yield an apparent rotational correlation time for the depolarization process, which, in most cases, is approximately the value expected for Brownian rotation of the entire protein. Apparent limiting fluorescence anisotropy values, which range from 0.20 to 0.32 for the proteins studied, are also obtained from the Perrin plots. The lower values for the limiting anisotropy found for some proteins are interpreted as indicating the existence of relatively rapid, limited (within a cone of angle 0 degrees--30 degrees) motion of the tryptophan side chains that is independent of the overall rotation of the protein. Examples of the use of this fluorescence technique to study protein conformational changes are presented, including the monomer in equilibrium dimer equilibrium of beta-lactoglobulin, the monomer in equilibrium tetramer equilibrium of melittin, the N in equilibrium F transition of human serum albumin, and the induced change in the conformation of cod parvalbumin caused by the removal of Ca+2. Because multitryptophan-containing proteins have certain tryptophans that are accessible to solute quencher and others that are inaccessible, this method can be used to determine the steady state anisotropy of each class of tryptophan residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdallah M. A., Biellmann J. F., Wiget P., Joppich-Kuhn R., Luisi P. L. Fluorescence quenching and energy transfer in complexes between horse-liver alcohol dehydrogenase and coenzymes. Eur J Biochem. 1978 Sep 1;89(2):397–405. doi: 10.1111/j.1432-1033.1978.tb12542.x. [DOI] [PubMed] [Google Scholar]
- Andrews L. J., Forster L. S. Protein difference spectra. Effect of solvent and charge on tryptophan. Biochemistry. 1972 May 9;11(10):1875–1879. doi: 10.1021/bi00760a023. [DOI] [PubMed] [Google Scholar]
- Belford G. G., Belford R. L., Weber G. Dynamics of fluorescence polarization in macromolecules. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1392–1393. doi: 10.1073/pnas.69.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H. E., Lehky P., Kohler L., Stein E. A., Fischer E. H. Comparative properties of vertebrate parvalbumins. J Biol Chem. 1977 May 10;252(9):2834–2838. [PubMed] [Google Scholar]
- Brochon J. C., Wahl P., Auchet J. C. Fluorescence time-resolved spectroscopy and fluorescence anisotropy decay of the Staphylococcus aureus endonuclease. Eur J Biochem. 1974 Feb 1;41(3):577–583. doi: 10.1111/j.1432-1033.1974.tb03299.x. [DOI] [PubMed] [Google Scholar]
- Brochon J. C., Wahl P. Measures des déclins de l'anisotropie de fluorescence de la gamma-globuline et de ses fragments Fab, Fc et F(ab) 2 marqués avec le 1-sulfonyl-5-diméthyl-aminonaphtalène. Eur J Biochem. 1972 Jan 31;25(1):20–32. doi: 10.1111/j.1432-1033.1972.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues and protein dynamics. Biochemistry. 1977 Dec 13;16(25):5546–5551. doi: 10.1021/bi00644a024. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Fluorescence quenching studies with proteins. Anal Biochem. 1981 Jul 1;114(2):199–227. doi: 10.1016/0003-2697(81)90474-7. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Jameson D. M. Acrylamide and oxygen fluorescence quenching studies with liver alcohol dehydrogenase using steady-state and phase fluorometry. Biochemistry. 1982 Aug 31;21(18):4443–4449. doi: 10.1021/bi00261a039. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Selvidge L. A. Fluorescence quenching of liver alcohol dehydrogenase by acrylamide. Biochemistry. 1982 Jan 5;21(1):117–125. doi: 10.1021/bi00530a021. [DOI] [PubMed] [Google Scholar]
- Formoso C., Forster L. S. Tryptophan fluorescence lifetimes in lysozyme. J Biol Chem. 1975 May 25;250(10):3738–3745. [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Sidechain torsional potentials and motion of amino acids in porteins: bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2002–2006. doi: 10.1073/pnas.72.6.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georghiou S., Thompson M., Mukhopadhyay A. K. Melittin-phospholipid interaction: evidence for melittin aggregation. Biochim Biophys Acta. 1981 Apr 6;642(2):429–432. doi: 10.1016/0005-2736(81)90458-2. [DOI] [PubMed] [Google Scholar]
- Ghiron C. A., Longworth J. W. Transfer of singlet energy within trypsin. Biochemistry. 1979 Aug 21;18(17):3828–3832. doi: 10.1021/bi00584a029. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. The fluorescence decay of tryptophan residues in native and denatured proteins. Biochim Biophys Acta. 1976 Apr 14;427(2):663–678. doi: 10.1016/0005-2795(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Hanson D. C., Yguerabide J., Schumaker V. N. Segmental flexibility of immunoglobulin G antibody molecules in solution: a new interpretation. Biochemistry. 1981 Nov 24;20(24):6842–6852. doi: 10.1021/bi00527a016. [DOI] [PubMed] [Google Scholar]
- Harvey S. C., Cheung H. C. Fluorescence depolarization studies on the flexibility of myosin rod. Biochemistry. 1977 Nov 29;16(24):5181–5187. doi: 10.1021/bi00643a004. [DOI] [PubMed] [Google Scholar]
- Hull W. E., Sykes B. D. Fluorotyrosine alkaline phosphatase: internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J Mol Biol. 1975 Oct 15;98(1):121–153. doi: 10.1016/s0022-2836(75)80105-7. [DOI] [PubMed] [Google Scholar]
- Imoto T., Forster L. S., Rupley J. A., Tanaka F. Fluorescence of lysozyme: emissions from tryptophan residues 62 and 108 and energy migration. Proc Natl Acad Sci U S A. 1972 May;69(5):1151–1155. doi: 10.1073/pnas.69.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Cherek H. Dipolar relaxation in proteins on the nanosecond timescale observed by wavelength-resolved phase fluorometry of tryptophan fluorescence. J Biol Chem. 1980 Feb 10;255(3):831–834. [PubMed] [Google Scholar]
- Lakowicz J. R., Freshwater G., Weber G. Nanosecond segmental mobilities of tryptophan residues in proteins observed by lifetime-resolved fluorescence anisotropies. Biophys J. 1980 Oct;32(1):591–601. doi: 10.1016/S0006-3495(80)84992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P., Cherek H., Balter A. Rotational freedom of tryptophan residues in proteins and peptides. Biochemistry. 1983 Apr 12;22(8):1741–1752. doi: 10.1021/bi00277a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Lipari G., Szabo A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys J. 1980 Jun;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy C., Holowka D. A., Cathou R. E. Nanosecond fluorescence spectroscopy of pyrenebutyrate anti-pyrene antibody complexes. Biochemistry. 1977 Aug 9;16(16):3668–3672. doi: 10.1021/bi00635a025. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Wolynes P. G., Karplus M. Picosecond dynamics of tyrosine side chains in proteins. Biochemistry. 1979 Mar 20;18(6):927–942. doi: 10.1021/bi00573a001. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov E. A., Yarmolenko V. V., Emelyanenko V. I., Burstein E. A., Closset J., Gerday C. Fluorescence studies of the calcium binding to whiting (Gadus merlangus) parvalbumin. Eur J Biochem. 1980 Aug;109(1):307–315. doi: 10.1111/j.1432-1033.1980.tb04796.x. [DOI] [PubMed] [Google Scholar]
- Ross J. B., Schmidt C. J., Brand L. Time-resolved fluorescence of the two tryptophans in horse liver alcohol dehydrogenase. Biochemistry. 1981 Jul 21;20(15):4369–4377. doi: 10.1021/bi00518a021. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Small E. W., Isenberg I. Hydrodynamic properties of a rigid molecule: rotational and linear diffusion and fluorescence anisotropy. Biopolymers. 1977 Sep;16(9):1907–1928. doi: 10.1002/bip.1977.360160907. [DOI] [PubMed] [Google Scholar]
- Talbot J. C., Dufourcq J., de Bony J., Faucon J. F., Lussan C. Conformational change and self association of monomeric melittin. FEBS Lett. 1979 Jun 1;102(1):191–193. doi: 10.1016/0014-5793(79)80957-6. [DOI] [PubMed] [Google Scholar]
- Teale F. W., Badley R. A. Depolarization of the intrinsic and extrinsic fluorescence of pepsinogen and pepsin. Biochem J. 1970 Feb;116(3):341–348. doi: 10.1042/bj1160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale F. W. Fluorescence depolarization by light-scattering in turbid solutions. Photochem Photobiol. 1969 Dec;10(6):363–374. doi: 10.1111/j.1751-1097.1969.tb05701.x. [DOI] [PubMed] [Google Scholar]
- Townend R., Herskovits T. T., Timasheff S. N., Gorbunoff M. J. The state of amino acid residues in beta-lactoglobulin. Arch Biochem Biophys. 1969 Feb;129(2):567–580. doi: 10.1016/0003-9861(69)90216-1. [DOI] [PubMed] [Google Scholar]
- Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem Photobiol. 1977 May;25(5):441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in tyrosine, tryptophan and related compounds. Biochem J. 1960 May;75:335–345. doi: 10.1042/bj0750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P., Weber G. Fluorescence depolarization of rabbit gamma globulin conjugates. J Mol Biol. 1967 Dec 14;30(2):371–382. doi: 10.1016/s0022-2836(67)80045-7. [DOI] [PubMed] [Google Scholar]
- Weber G., Shinitzky M. Failure of Energy Transfer between Identical Aromatic Molecules on Excitation at the Long Wave Edge of the Absorption Spectrum. Proc Natl Acad Sci U S A. 1970 Apr;65(4):823–830. doi: 10.1073/pnas.65.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltman J. K., Edelman G. M. Fluorescence polarization of human gamma-G-immunoglobulins. Biochemistry. 1967 May;6(5):1437–1447. doi: 10.1021/bi00857a028. [DOI] [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]


