Abstract
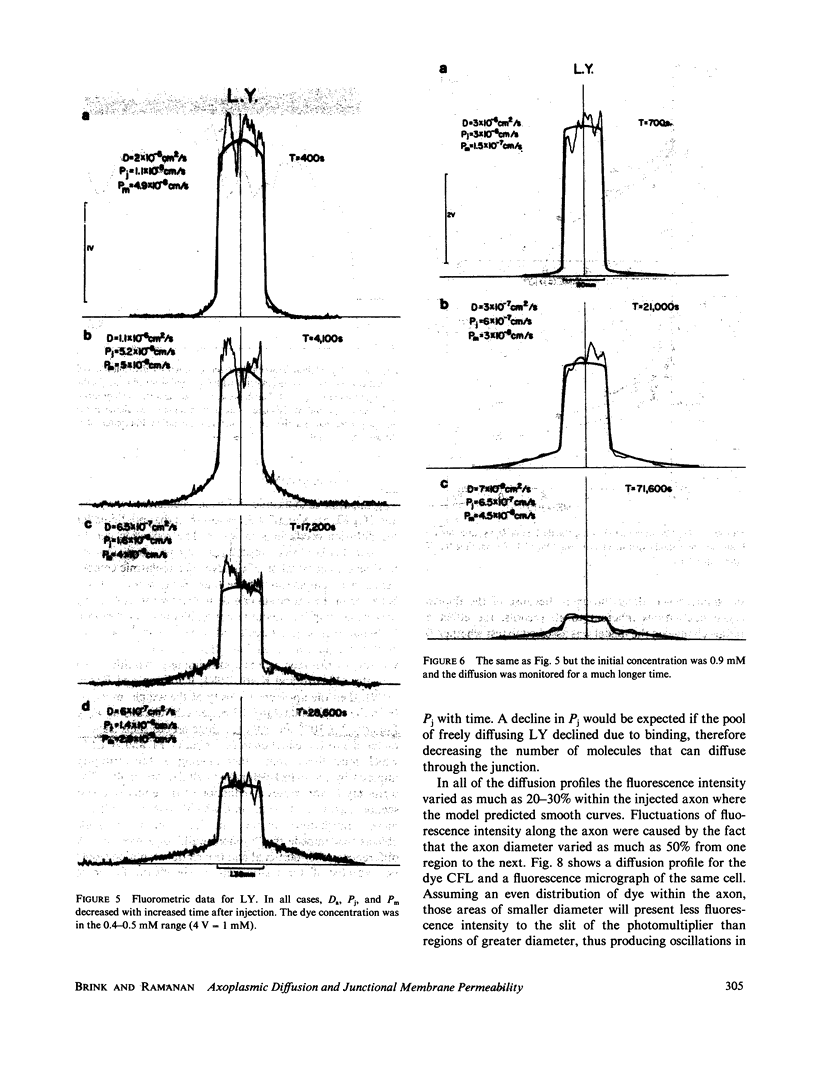
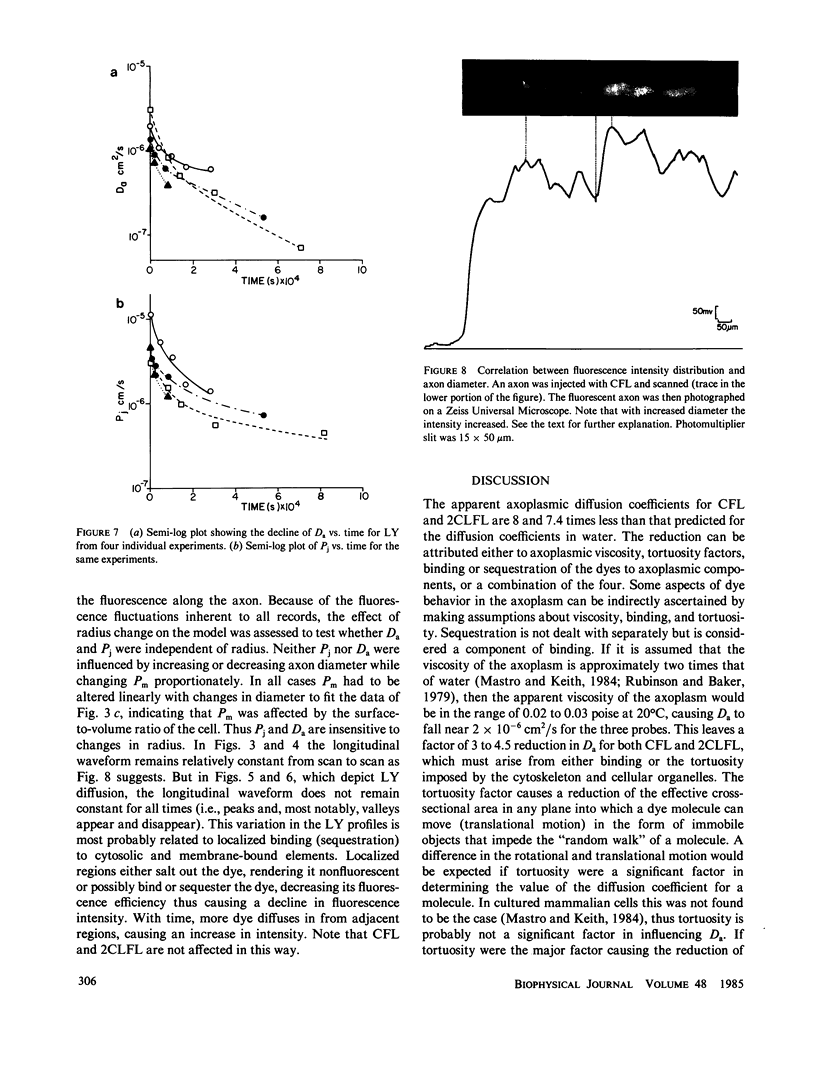
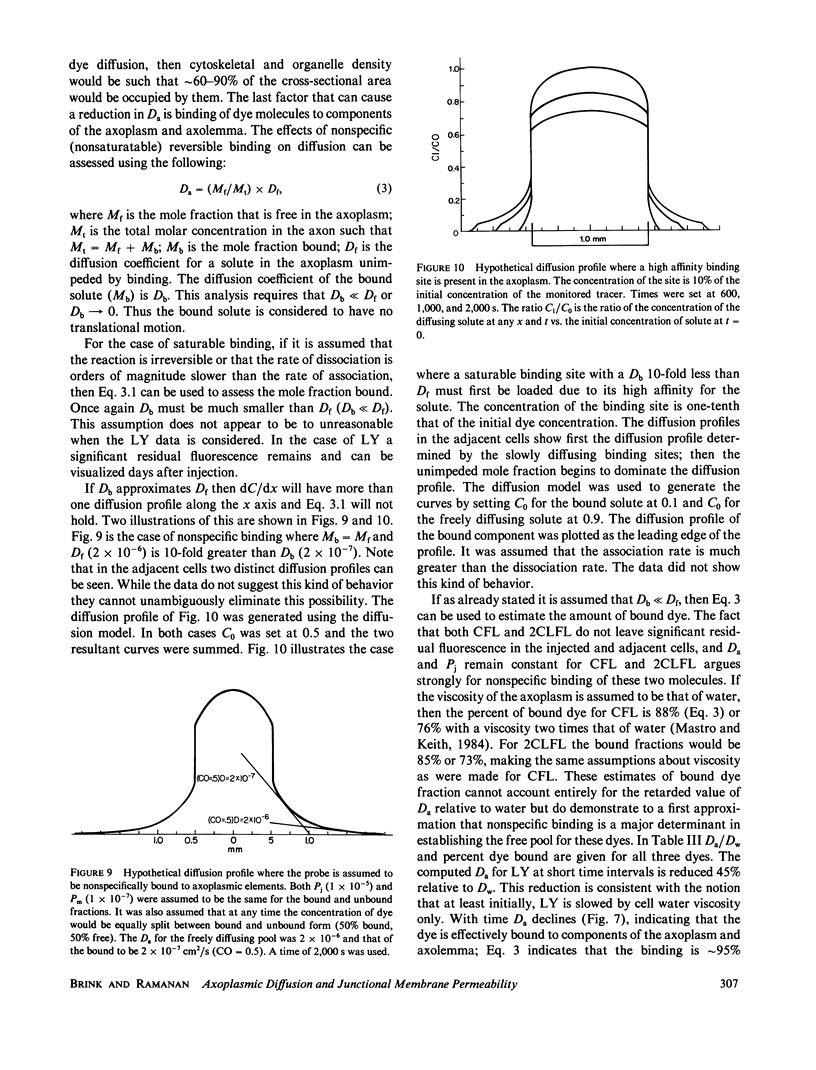
The diffusion of the three fluorescent probes dichlorofluorescein, carboxyfluorescein, and Lucifer Yellow within the septate median giant axon of the earthworm was monitored using fluorometric methods. A diffusion model was derived that allowed computation of the apparent axoplasmic diffusion coefficient, junctional membrane permeability (septal membranes), and plasma membrane permeability for each probe. Dichlorofluorescein and carboxyfluorescein have similar apparent axoplasmic diffusion coefficients, which were reduced by a factor of eight relative to that predicted from the Einstein-Stokes equation. Nonspecific reversible binding appears to be the major cause of the retarded diffusion coefficients. Junctional membrane permeability for dichlorofluorescein was 4.7 to 73-fold greater than that for carboxyfluorescein. This difference could not be explained on the basis of molecular size but can be explained by the difference in charge between the two molecules. Diffusion coefficients and junctional membrane permeabilities remained constant with time for both dyes. The diffusion of Lucifer Yellow within the axoplasm and permeability through the junctional membranes did not remain constant with time but declined. From this it was inferred that Lucifer Yellow experienced a slow, irreversible binding to axoplasmic elements. All three probes had finite plasma membrane permeabilities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada Y., Bennett M. V. Experimental alteration of coupling resistance at an electrotonic synapse. J Cell Biol. 1971 Apr;49(1):159–172. doi: 10.1083/jcb.49.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink P. R., Dewey M. M., Colflesh D. E., Kensler R. W. Polymorphic nexuses in the earthworm Lumbricus terrestris. J Ultrastruct Res. 1981 Dec;77(3):233–240. doi: 10.1016/s0022-5320(81)80021-4. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Dewey M. M. Evidence for fixed charge in the nexus. Nature. 1980 May 8;285(5760):101–102. doi: 10.1038/285101a0. [DOI] [PubMed] [Google Scholar]
- Brink P. R. Effect of deuterium oxide on junctional membrane channel permeability. J Membr Biol. 1983;71(1-2):79–87. doi: 10.1007/BF01870676. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Verselis V., Barr L. Solvent-Solute Interactions within the Nexal Membrane. Biophys J. 1984 Jan;45(1):121–124. doi: 10.1016/S0006-3495(84)84133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink P., Barr L. The resistance of the septum of the median giant axon of the earthworm. J Gen Physiol. 1977 May;69(5):517–536. doi: 10.1085/jgp.69.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokelet G. R., Meiselman H. J. Rheological comparison of hemoglobin solutions and erythrocyte suspensions. Science. 1968 Oct 11;162(3850):275–277. doi: 10.1126/science.162.3850.275. [DOI] [PubMed] [Google Scholar]
- Dintenfass L. Internal viscosity of the red cell and a blood viscosity equation. Nature. 1968 Aug 31;219(5157):956–958. doi: 10.1038/219956a0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. The structure and permeability of isolated hepatocyte gap junctions. Cold Spring Harb Symp Quant Biol. 1976;40:37–43. doi: 10.1101/sqb.1976.040.01.006. [DOI] [PubMed] [Google Scholar]
- Günther J. Neuronal syncytia in the giant fibres of earthworms. J Neurocytol. 1975 Feb;4(1):55–62. doi: 10.1007/BF01099095. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Brink P. R., Dewey M. M. The septum of the lateral axon of the earthworm: a thin section and freeze-fracture study. J Neurocytol. 1979 Oct;8(5):565–590. doi: 10.1007/BF01208510. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeable junctions. Cold Spring Harb Symp Quant Biol. 1976;40:49–63. doi: 10.1101/sqb.1976.040.01.008. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Baker T. S., Goodenough D. A. Gap junction structures. VI. Variation and conservation in connexon conformation and packing. Biophys J. 1984 Jan;45(1):208–218. doi: 10.1016/S0006-3495(84)84149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson K. A., Baker P. F. The flow properties of axoplasm in a defined chemical environment: influence of anions and calcium. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):323–345. doi: 10.1098/rspb.1979.0068. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis V., Brink P. R. Voltage clamp of the earthworm septum. Biophys J. 1984 Jan;45(1):147–150. doi: 10.1016/S0006-3495(84)84143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R. The permeability to tetraethylammonium ions of the surface membrane and the intercalated disks of sheep and calf myocardium. J Physiol. 1974 Aug;240(3):741–762. doi: 10.1113/jphysiol.1974.sp010632. [DOI] [PMC free article] [PubMed] [Google Scholar]