Abstract
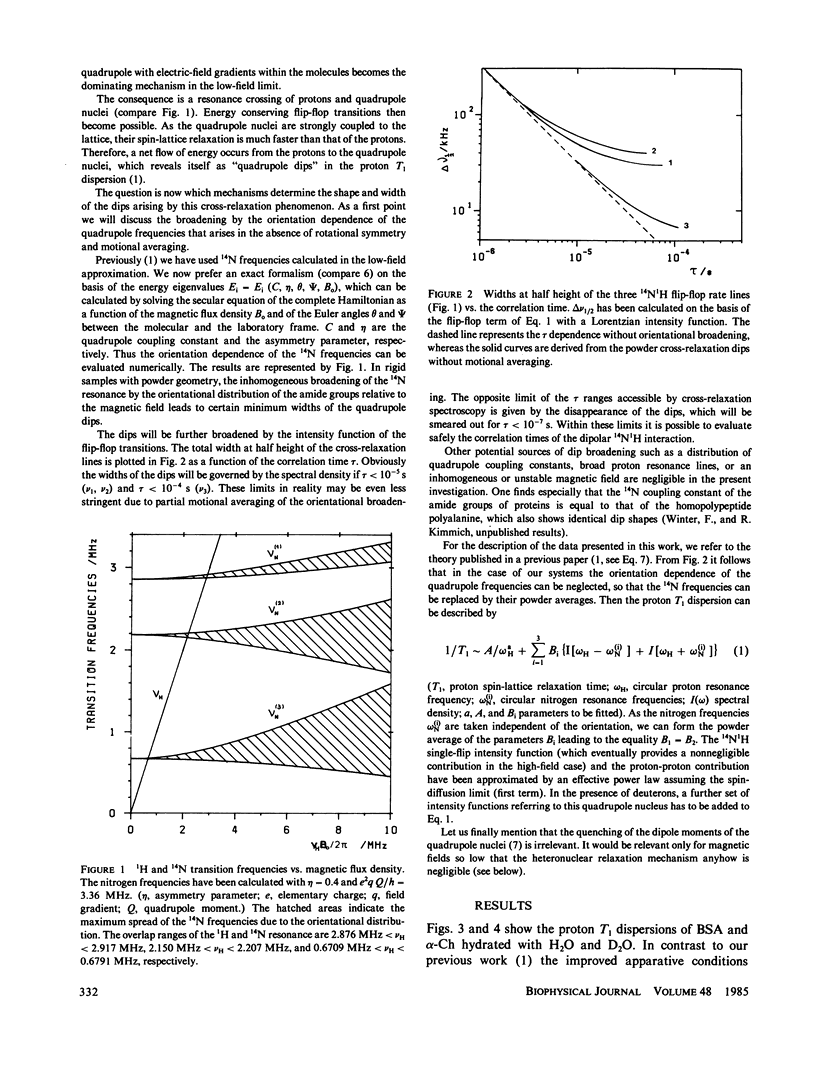
The frequency dependence of the proton spin-lattice relaxation time T1 of solid hydrated bovine serum albumin and alpha-chymotrypsin has been measured over 4.5 decades in the range 10(4) to 3 X 10(8) Hz mainly by the aid of the field-cycling technique. The comparison between H2O- and D2O-hydrated samples permitted the distinction of exchangeable and unexchangeable protons. In all cases the 14N1H cross-relaxation dips due mainly to the amide groups have been observed. In addition, in the case of the deuterium exchanged proteins a 2H1H quadrupole dip appears. The amide groups act as relaxation sinks due to the coupling of the amide proton to 14N and adjacent protons. Outside of the dip regions the proton-proton coupling dominates. The fluctuations of the 14N1H and 1H1H interactions are of a different type. The unexchangeable protons show a T1 dispersion outside of the quadrupole dip regions given by the exceptional power law T1 approximately v0.75 +/- 0.05. It is shown that apart from structural information of the 14N spectra, 14N1H cross-relaxation spectroscopy permits the determination of correlation times in the range 10(-7) s less than tau less than 10(-4)S.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Kallenbach N. R. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1983 Nov;16(4):521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- Escanye J. M., Canet D., Robert J. Frequency dependence of water proton longitudinal NMR relaxation times in mouse tissues around the freezing transition. Biochim Biophys Acta. 1983 Jun 2;762(3):445–451. doi: 10.1016/0167-4889(83)90010-1. [DOI] [PubMed] [Google Scholar]
- Kimmich R., Nusser W., Winter F. In vivo NMR field-cycling relaxation spectroscopy reveals 14N1H relaxation sinks in the backbones of proteins. Phys Med Biol. 1984 May;29(5):593–596. doi: 10.1088/0031-9155/29/5/011. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Bryant R. G., Hallenga K., Jacob G. S. Magnetic cross-relaxation among protons in protein solutions. Biochemistry. 1978 Oct 3;17(20):4348–4358. doi: 10.1021/bi00613a037. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. I. Apotransferrin. J Biol Chem. 1969 Jun 25;244(12):3283–3289. [PubMed] [Google Scholar]
- Schramm S., Oldfield E. Nuclear magnetic resonance studies of amino acids and proteins. Rotational correlation times of proteins by deuterium nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Jun 7;22(12):2908–2913. doi: 10.1021/bi00281a020. [DOI] [PubMed] [Google Scholar]
- Spohn K. H., Kimmich R. Characterization of the mobility of various chemical groups in the purple membrane of Halobacterium halobium by 13C, 31P and 2H solid state NMR. Biochem Biophys Res Commun. 1983 Jul 29;114(2):713–720. doi: 10.1016/0006-291x(83)90839-2. [DOI] [PubMed] [Google Scholar]


