Abstract
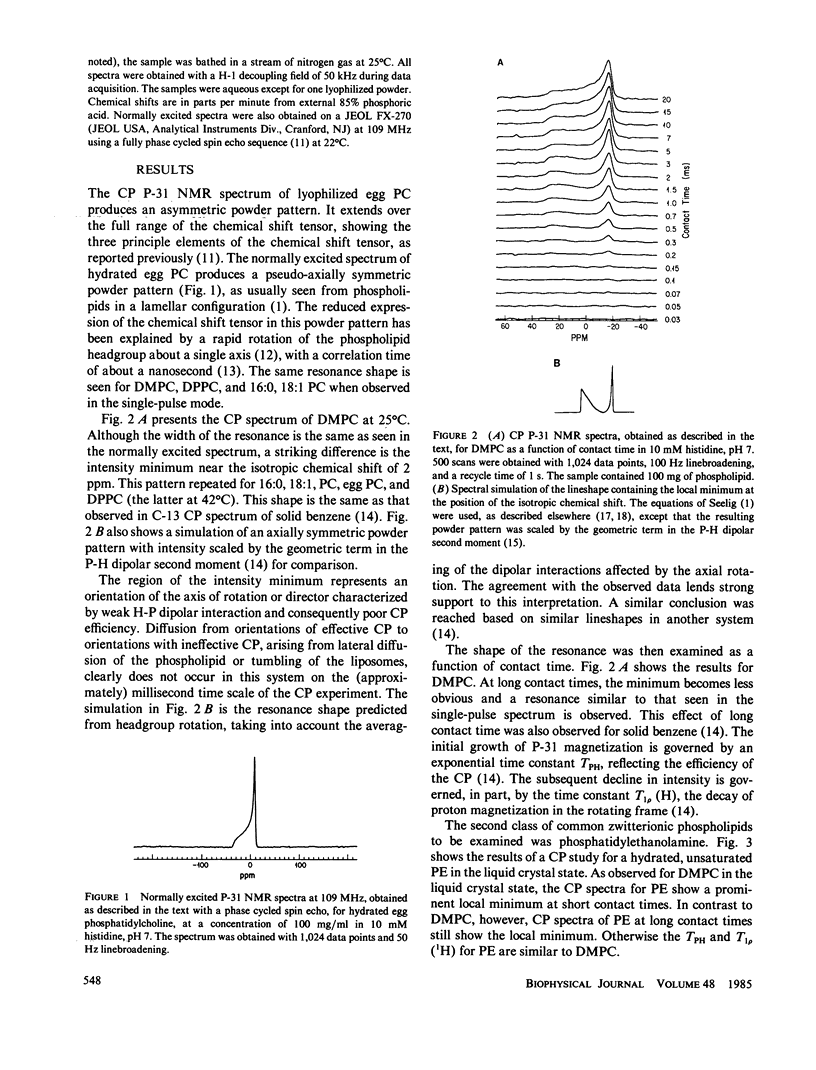
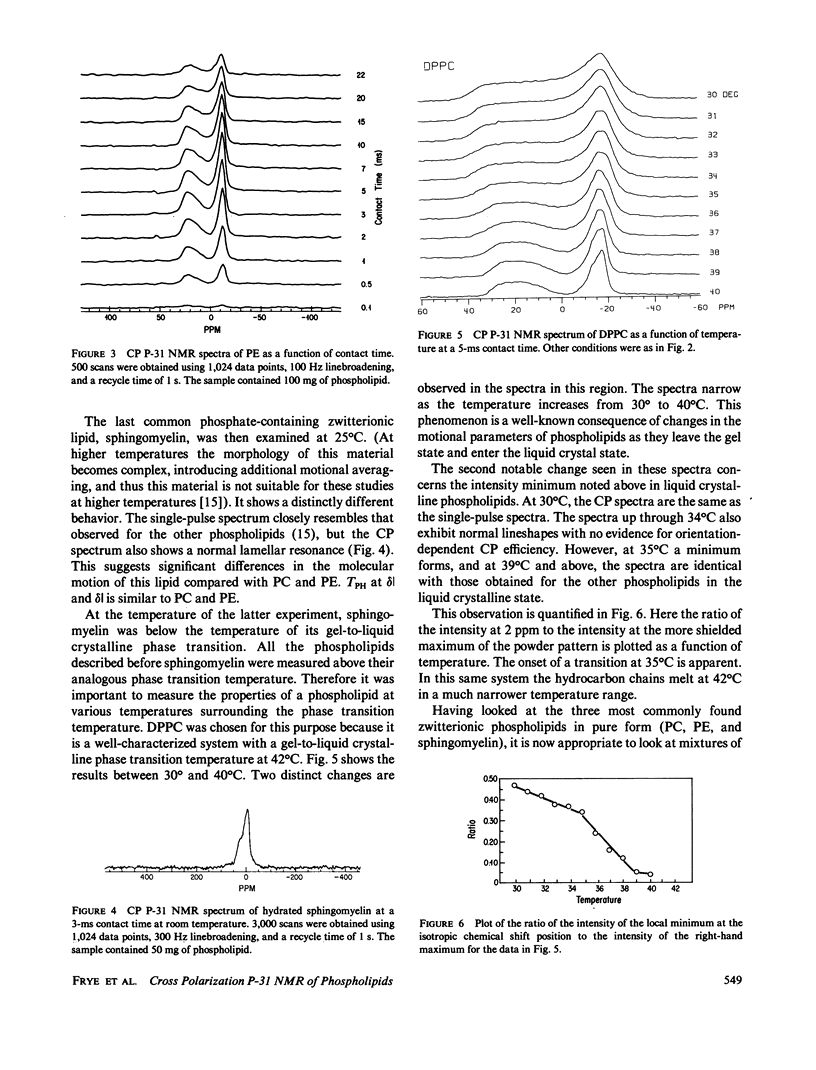
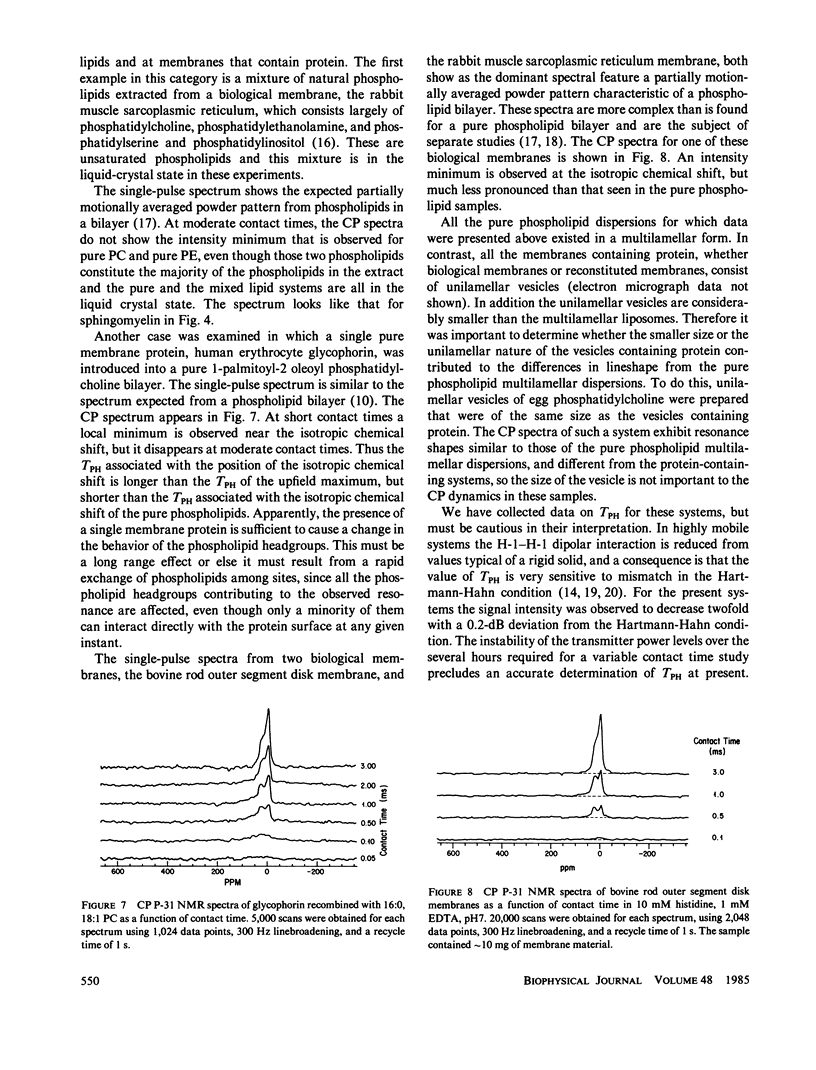
P-31 single-pulse and cross-polarization (CP) nuclear magnetic resonance spectra were obtained of aqueous dispersions of pure phospholipids. Dimyristoyl phosphatidylcholine, dipalmitoylphosphatidylcholine, 1-palmitoyl-2-oleoyl phosphatidylcholine, egg phosphatidylcholine, bovine brain sphingomyelin, and transphosphatidylated (from egg phosphatidylcholine) phosphatidylethanolamine were studied. The spectra from all the phospholipids, taken in the usual single-pulse mode, showed the pseudo-axially symmetric powder pattern typical of phospholipids in a hydrated lamellar form. P-31 CP spectra of all the phosphatidylcholines and phosphatidylethanolamine revealed a decrease in intensity in the vicinity of the isotropic chemical shift as long as the lipid was above the gel-to-liquid crystalline phase transition temperature. This intensity pattern has been observed previously for C-13 CP spectra of molecules rotating rapidly about a single well-defined axis (e.g., solid benzene) (Pines, A., M.G. Gibby, and J.S. Waugh, 1973, J. Chem. Phys., 59:569-590). Pure lipid dispersions below their gel-to-liquid crystalline phase transition temperature, including dipalmitoylphosphatidylcholine and sphingomyelin, do not exhibit a local minimum in the CP spectrum at the position of the isotropic chemical shift. Thus, below the phase transition temperature, there is not the same rapid rotation of the headgroup about a well-defined axis. A dramatic change in the rate of headgroup rotation is shown to take place at the pretransition of dipalmitoylphosphatidylcholine. P-31 CP spectra were also obtained for bovine rod outer segment disk membranes, rabbit muscle sarcoplasmic reticulum membranes, a total lipid extract of the latter, and a recombined membrane containing human erythrocyte glycophorin. The CP spectra were similar to the single-pulse spectra, indicating a substantial difference in behavior from pure phospholipid dispersions. This is interpreted in terms of a slower headgroup rotation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A. D., Lund M., Yeagle P. L. Evidence for the influence of the protein-phospholipid interface on sarcoplasmic reticulum Ca++ Mg++ ATPase activity. Biophys J. 1981 Nov;36(2):393–407. doi: 10.1016/S0006-3495(81)84739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert A. D., Yeagle P. L. Phospholipid domains in bovine retinal rod outer segment disk membranes. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7188–7191. doi: 10.1073/pnas.80.23.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Letter: Observation of the effect of water on the 31P nuclear magnetic resonance spectra of dipalmitoyllecithin. J Am Chem Soc. 1976 Feb 4;98(3):851–853. doi: 10.1021/ja00419a044. [DOI] [PubMed] [Google Scholar]
- Herbette L., Marquardt J., Scarpa A., Blasie J. K. A direct analysis of lamellar x-ray diffraction from hydrated oriented multilayers of fully functional sarcoplasmic reticulum. Biophys J. 1977 Nov;20(2):245–272. doi: 10.1016/S0006-3495(77)85547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Yeagle P. L. Temperature-dependent morphological and phase behavior of sphingomyelin. Biochim Biophys Acta. 1980 Sep 18;601(2):271–281. doi: 10.1016/0005-2736(80)90532-5. [DOI] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. Orientation and dynamics of phospholipid head groups in bilayers and membranes determined from 31P nuclear magnetic resonance chemical shielding tensors. Biochemistry. 1977 Feb 8;16(3):519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally H. Investigation of phosphatidylethanolamine bilayers by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1976 Nov 30;15(24):5199–5204. doi: 10.1021/bi00669a001. [DOI] [PubMed] [Google Scholar]
- Selinsky B. S., Yeagle P. L. Two populations of phospholipids exist in sarcoplasmic reticulum and in recombined membranes containing Ca-ATPase. Biochemistry. 1984 May 8;23(10):2281–2288. doi: 10.1021/bi00305a030. [DOI] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975 Mar;20(3):211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Huang C. H., Martin R. B. Headgroup conformation and lipid--cholesterol association in phosphatidylcholine vesicles: a 31P(1H) nuclear Overhauser effect study. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3477–3481. doi: 10.1073/pnas.72.9.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L., Romans A. Y. The glycophorin-phospholipid interface in recombined systems. A 31P-nuclear magnetic resonance study. Biophys J. 1981 Feb;33(2):243–252. doi: 10.1016/S0006-3495(81)84885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


