Abstract
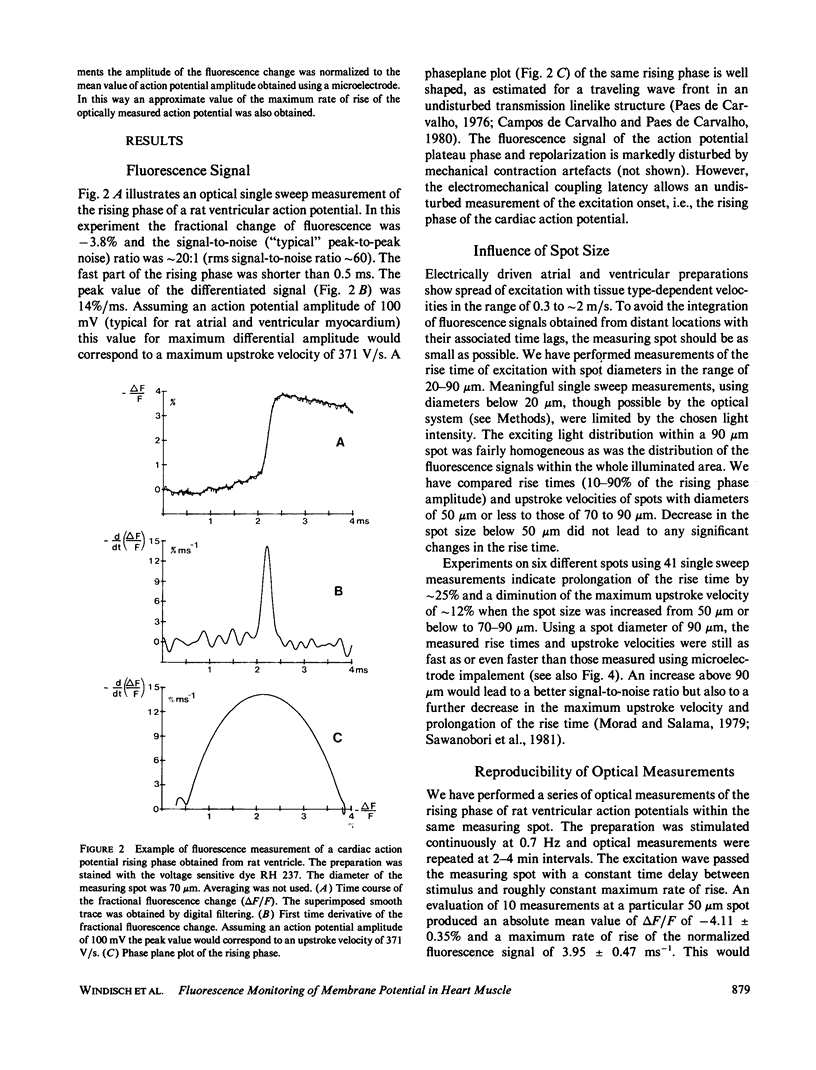
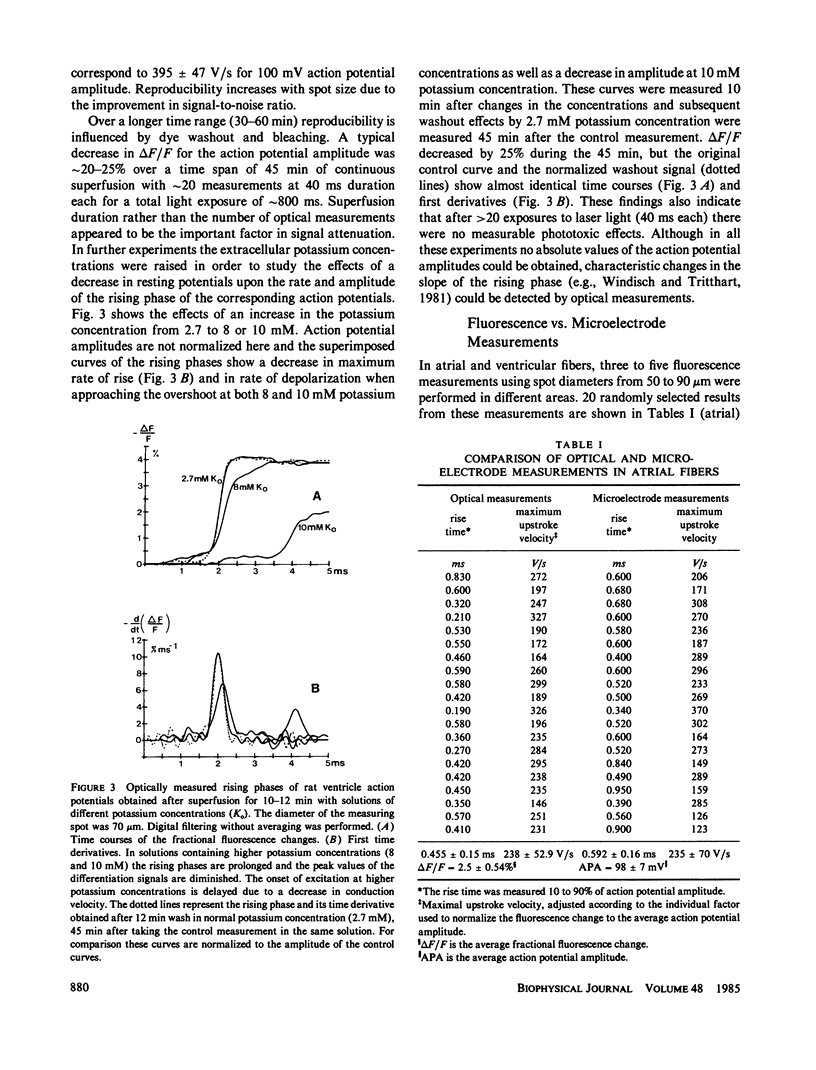
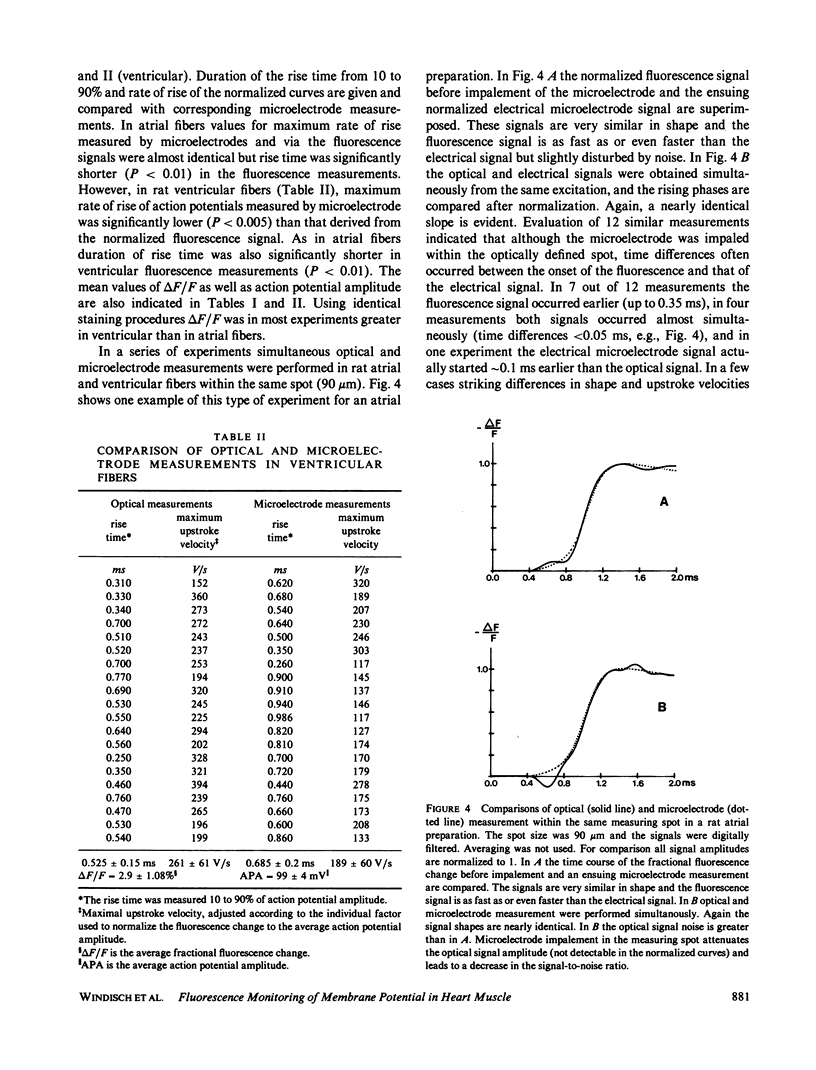
The rising phase of rat cardiac action potentials was measured in physiological solutions using the voltage-sensitive dye RH 237. A newly designed optical system and an argon ion laser for excitation allowed measurements without averaging over small areas (20-90 microns diameter) with high time resolution (response time 10-90%, 0.12 ms). The mean value of the fractional change in the fluorescence signal was approximately 3%/100 mV. The signal-to-noise ratio was approximately 60 rms (spot diameter 70 microns) allowing signal differentiation after digital filtering. Multiple measurements within the same spot showed a decrease in the fractional fluorescence change of 20 to 25% after 45 min without changes in the shape of the rising phase and with no measureable phototoxic effects. The optically measured rising phases showed rise times significantly (P less than 0.01) shorter and maximum upstroke velocities equal to or most often greater than those obtained with microelectrode techniques. Comparing simultaneous optical and electrical measurements within the same spot the microelectrode signal was often slightly delayed. This refined system seems well suited to detect fast cellular electrical activities with time and space resolutions comparable or even superior to those obtained using microelectrode techniques.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Davila H. V., Salzberg B. M., Cohen L. B., Waggoner A. S. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol. 1973 Jan 31;241(109):159–160. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- Dillon S., Morad M. A new laser scanning system for measuring action potential propagation in the heart. Science. 1981 Oct 23;214(4519):453–456. doi: 10.1126/science.6974891. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Rich T. L., Beydler S., Kreman M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ Res. 1982 Aug;51(2):117–130. doi: 10.1161/01.res.51.2.117. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981 Feb;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: evidence for electrical activity. J Physiol. 1980 Jul;304:503–518. doi: 10.1113/jphysiol.1980.sp013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Fine A., Farber I. C., Hildesheim R. Fluorescence monitoring of electrical responses from small neurons and their processes. Biophys J. 1983 May;42(2):195–198. doi: 10.1016/S0006-3495(83)84386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Hildesheim R., Farber I. C., Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982 Sep;39(3):301–308. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Hill B. C., Courtney K. R. Voltage-sensitive dyes. Discerning contraction and electrical signals in myocardium. Biophys J. 1982 Dec;40(3):255–257. doi: 10.1016/S0006-3495(82)84481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Fujii S., Kamino K. Optical monitoring of spontaneous electrical activity of 8-somite embryonic chick heart. Jpn J Physiol. 1979;29(5):635–639. doi: 10.2170/jjphysiol.29.635. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Cohen L. B., Salzberg B. M., Obaid A. L., Bezanilla F. Charge-shift probes of membrane potential. Characterization of aminostyrylpyridinium dyes on the squid giant axon. Biophys J. 1985 Jan;47(1):71–77. doi: 10.1016/S0006-3495(85)83878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Salama G. Optical probes of membrane potential in heart muscle. J Physiol. 1979 Jul;292:267–295. doi: 10.1113/jphysiol.1979.sp012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Davila H. V. A large change in dye absorption during the action potential. Biophys J. 1974 Dec;14(12):983–986. doi: 10.1016/S0006-3495(74)85963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Salama G., Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976 Feb 6;191(4226):485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Sawanobori T., Hirota A., Fujii S., Kamino K. Optical recording of conducted action potential in heart muscle using a voltage-sensitive dye. Jpn J Physiol. 1981;31(3):369–380. doi: 10.2170/jjphysiol.31.369. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J., Janse M. J. Fine structural identification of individual cells subjected to microelectrode recording in perfused cardiac preparations. J Mol Cell Cardiol. 1982 Apr;14(4):233–247. doi: 10.1016/0022-2828(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Tritthart H., MacLeod D. P., Stierle H. E., Krause H. Effects of Ca-free and EDTA-containing tyrode solution on transmembrane electrical activity and contraction in guinea pig papillary muscle. Pflugers Arch. 1973;338(4):361–376. doi: 10.1007/BF00586077. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. Dye indicators of membrane potential. Annu Rev Biophys Bioeng. 1979;8:47–68. doi: 10.1146/annurev.bb.08.060179.000403. [DOI] [PubMed] [Google Scholar]
- Walton M., Fozzard H. A. The relation of Vmax to INa, GNa, and h infinity in a model of the cardiac Purkinje fiber. Biophys J. 1979 Mar;25(3):407–420. doi: 10.1016/S0006-3495(79)85312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- Windisch H., Tritthart H. A. Calcium ion effects on the rising phases of action potentials obtained from guinea-pig papillary muscles at different potassium concentrations. J Mol Cell Cardiol. 1981 May;13(5):457–469. doi: 10.1016/0022-2828(81)90263-7. [DOI] [PubMed] [Google Scholar]
- de Carvalho A. C., de Carvalho A. P. Phase plane analysis of uniform and non-uniform propagation of activity in a model of squid giant axon. An Acad Bras Cienc. 1980 Dec;52(4):893–902. [PubMed] [Google Scholar]


