Abstract
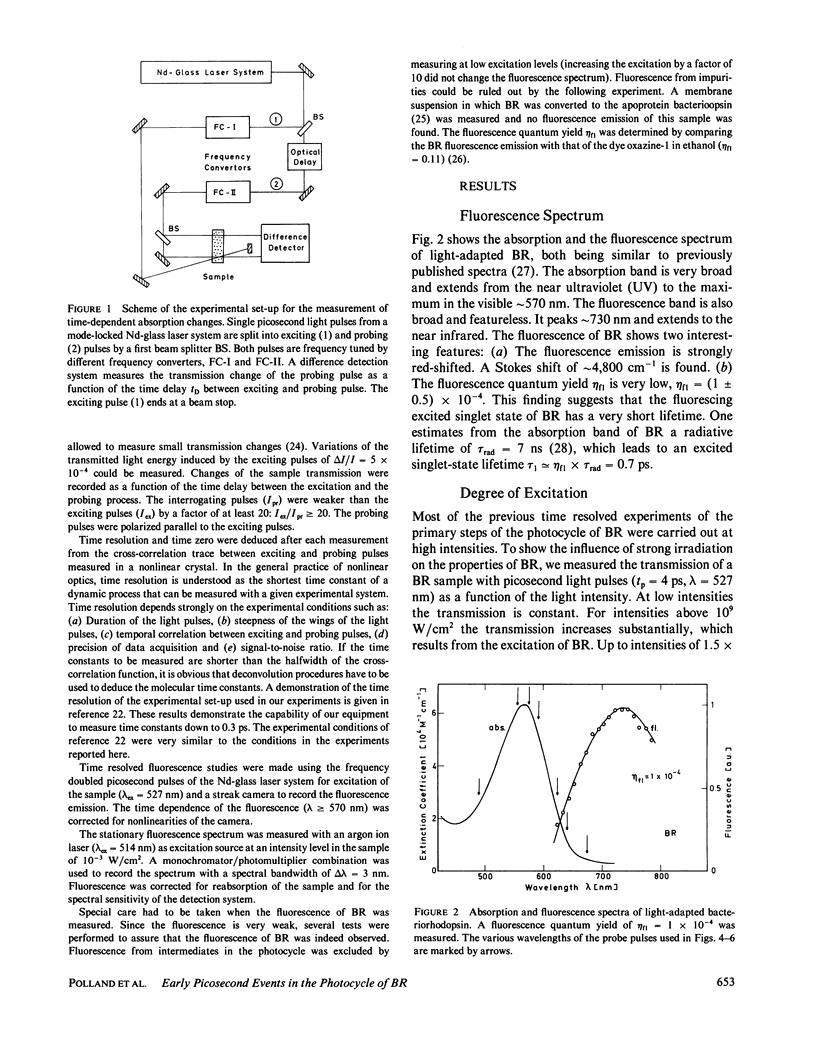
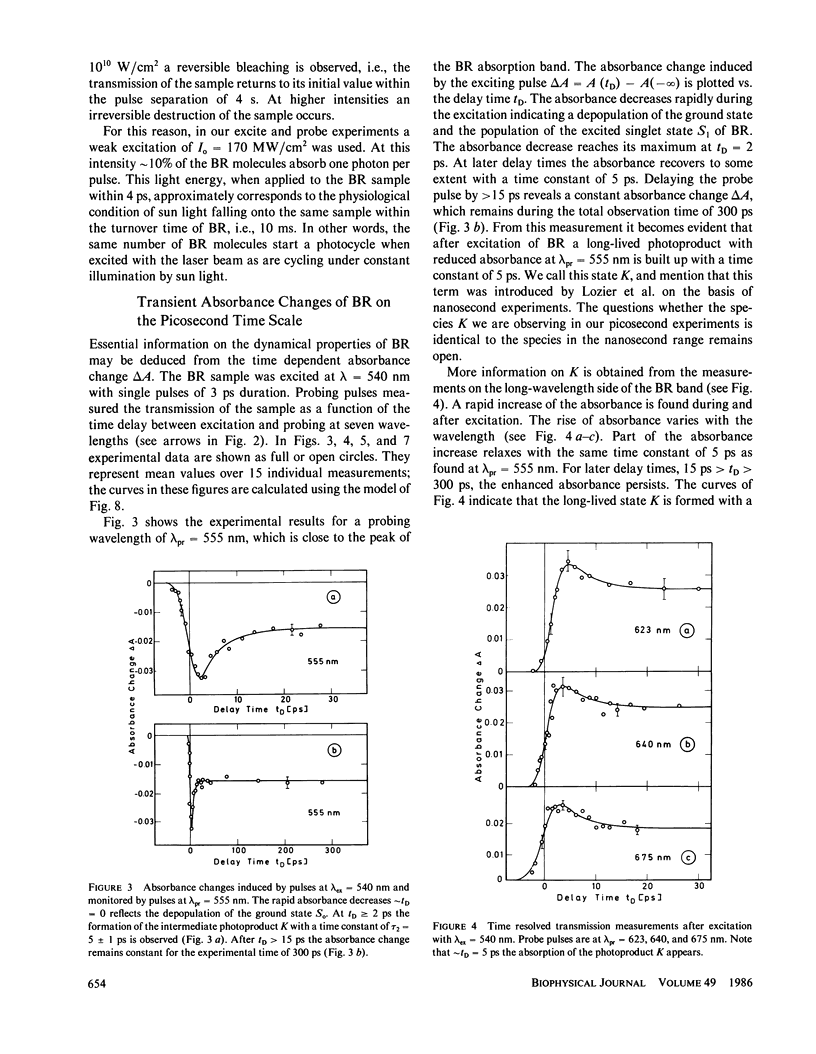
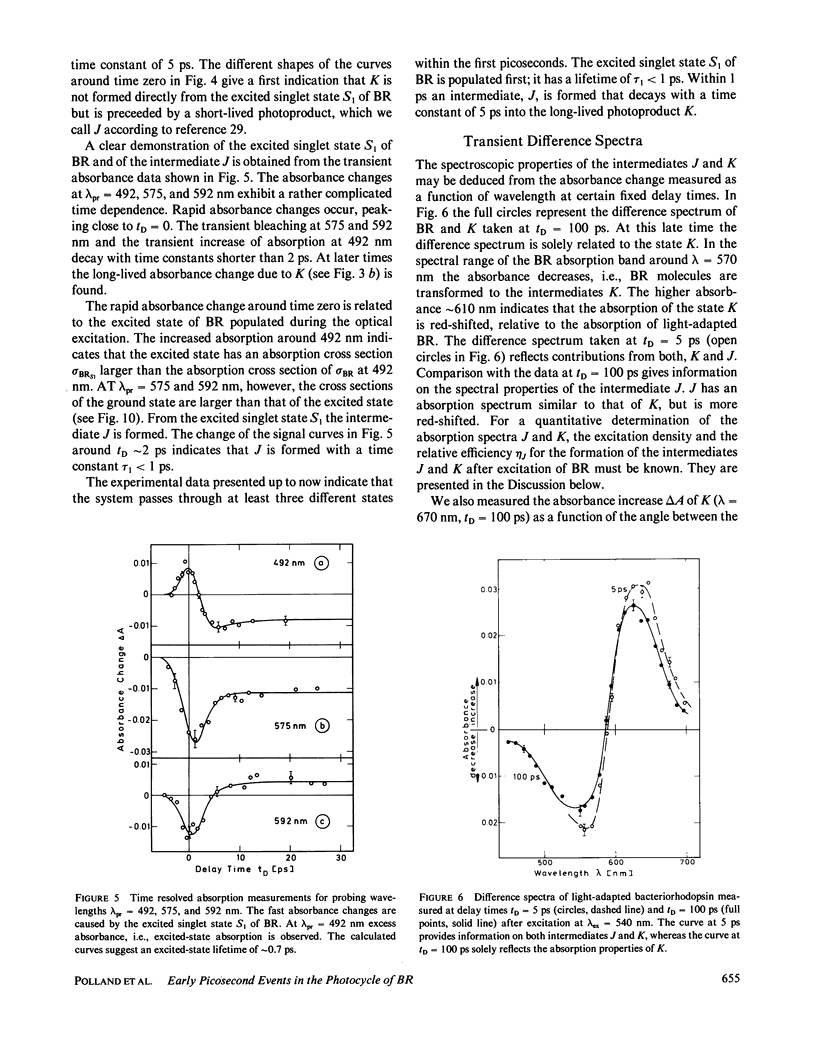
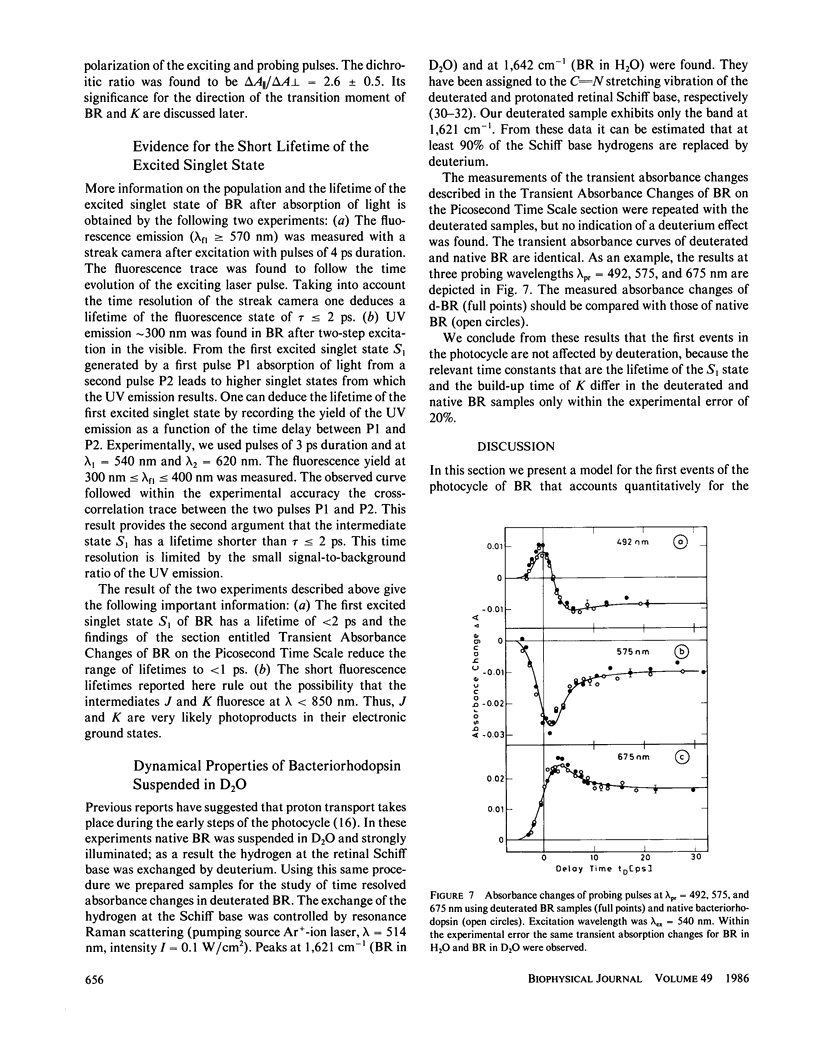
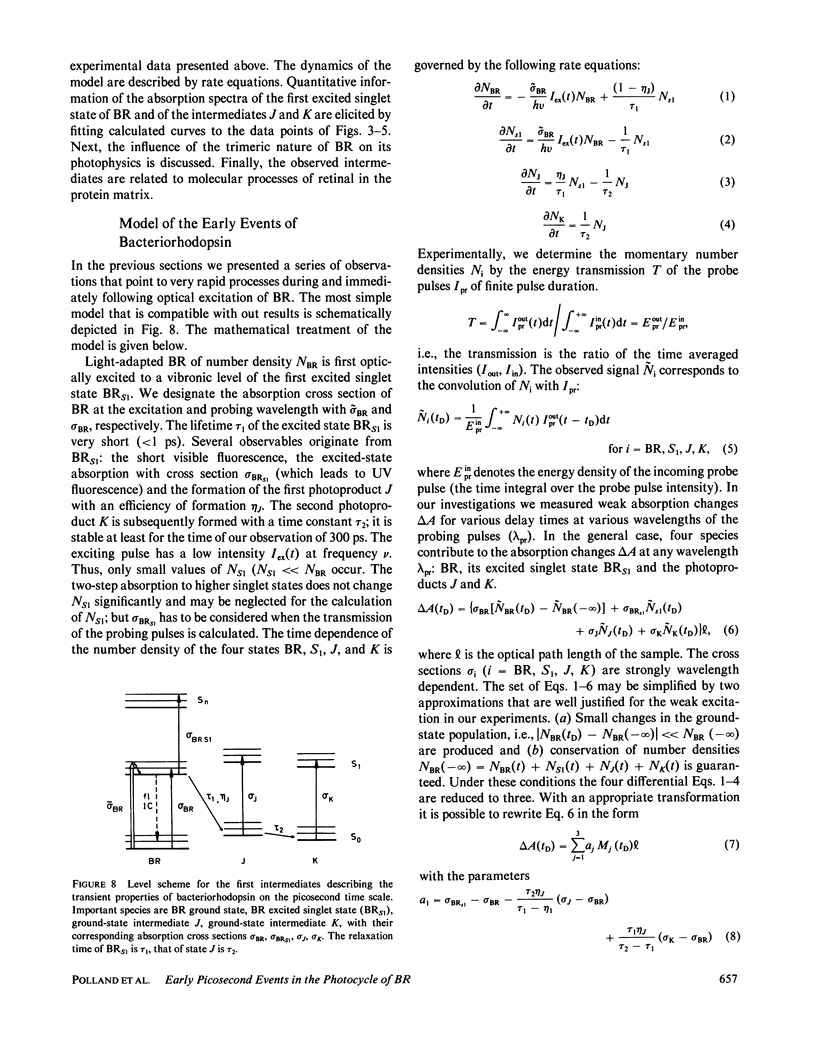
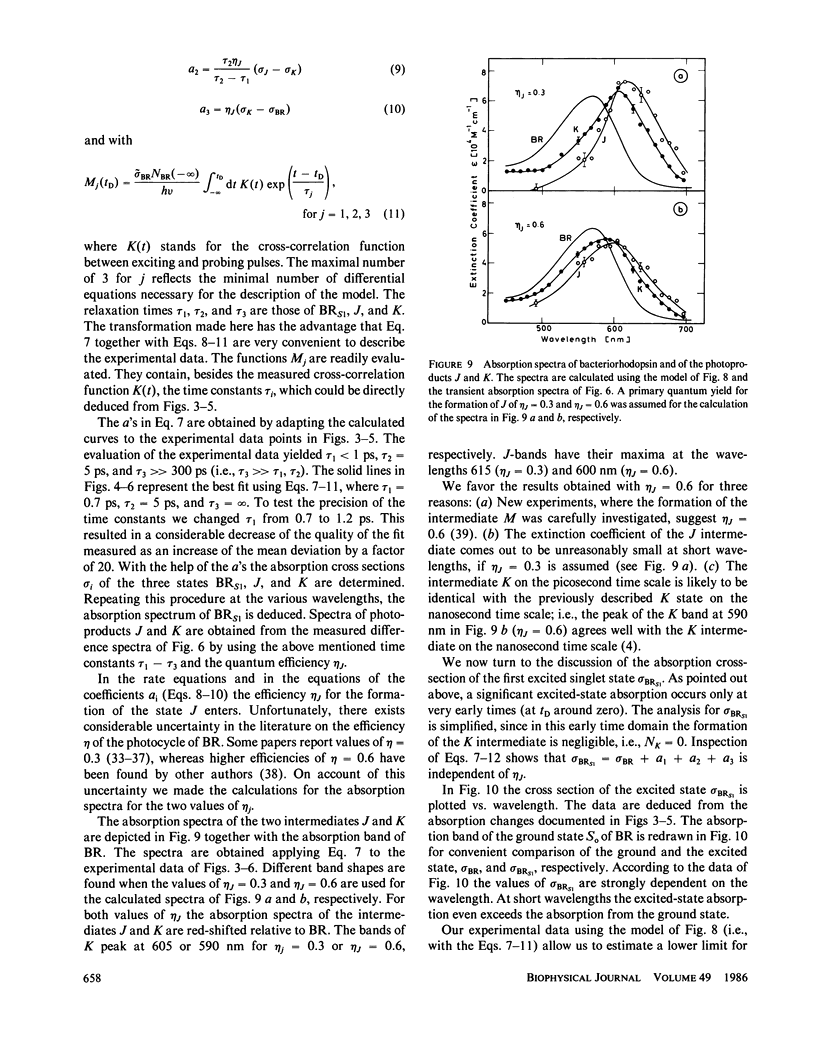
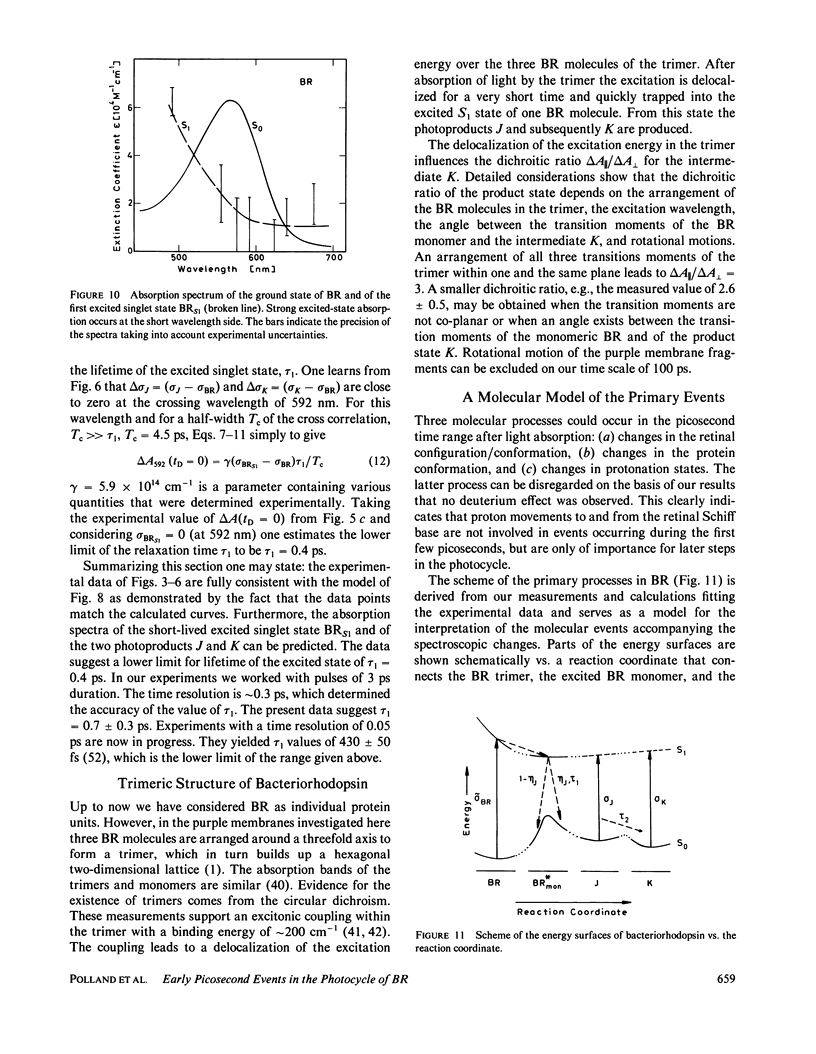
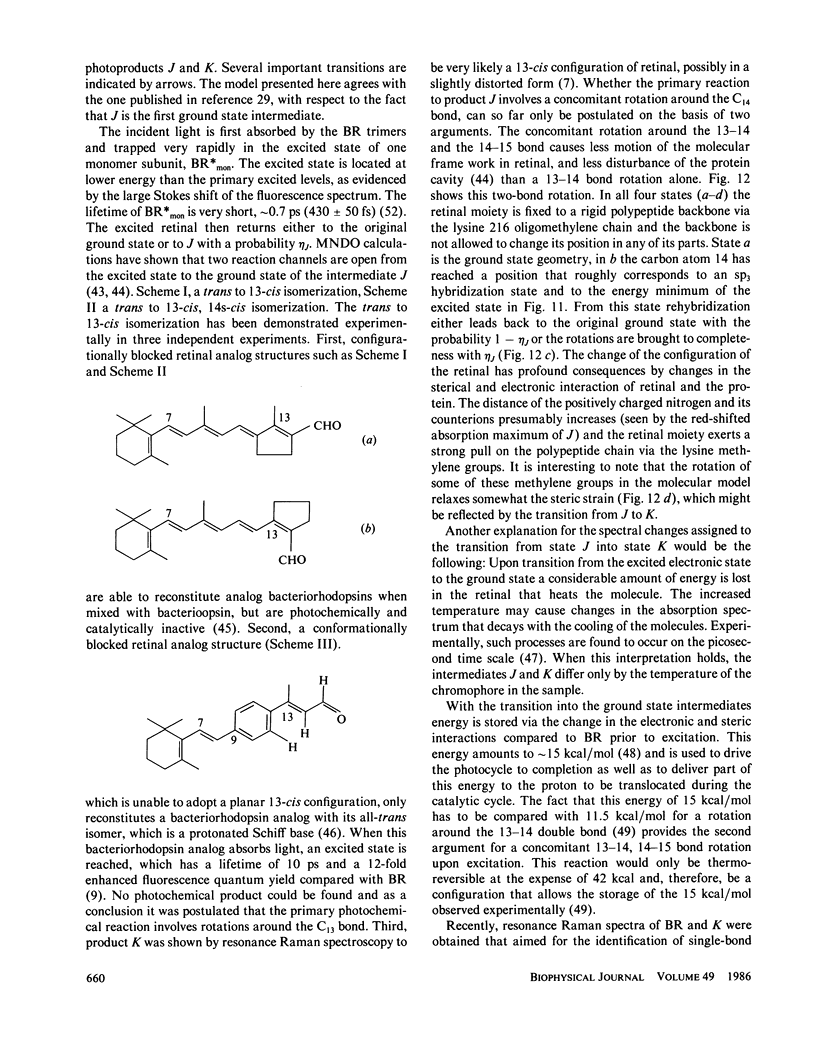
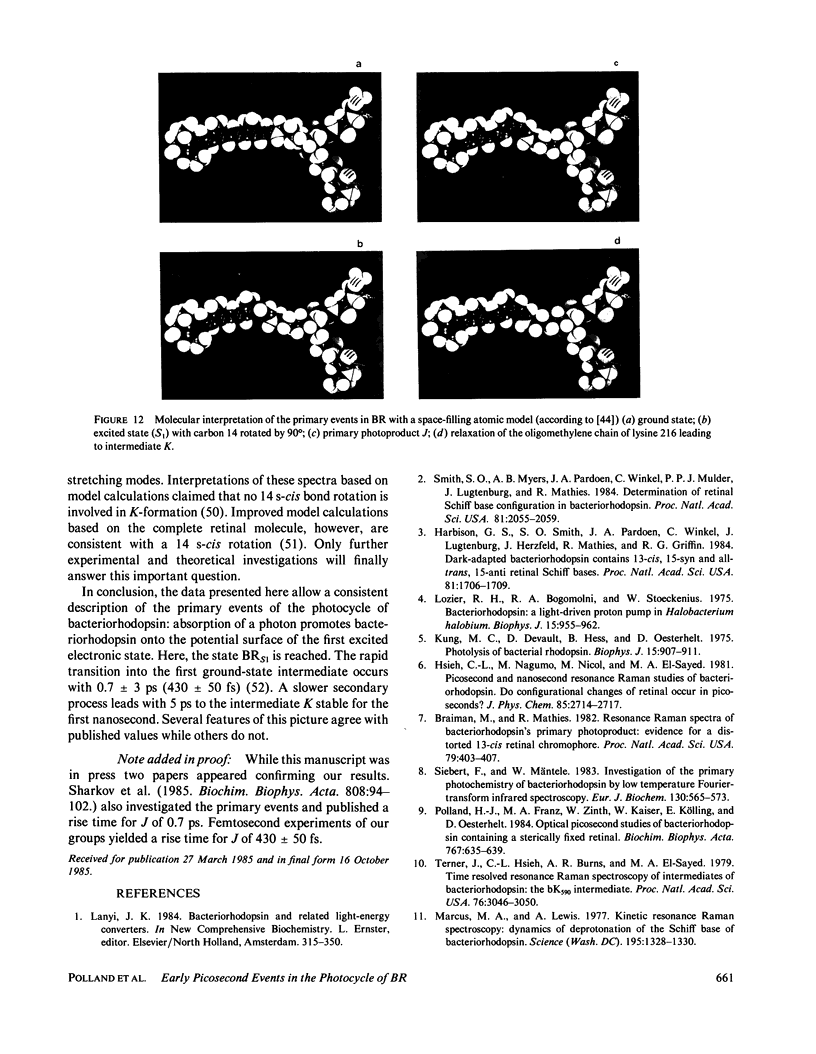
The primary processes of the photochemical cycle of light-adapted bacteriorhodopsin (BR) were studied by various experimental techniques with a time resolution of 5 × 10-13 s. The following results were obtained. (a) After optical excitation the first excited singlet state S1 of bacteriorhodopsin is observed via its fluorescence and absorption properties. The population of the excited singlet state decays with a lifetime τ1 of ∼0.7 ps (430 ± 50 fs) (52). (b) With the same time constant the first ground-state intermediate J builds up. Its absorption spectrum is red-shifted relative to the spectrum of BR by ∼30 nm. (c) The second photoproduct K, which appears with a time constant of τ2 = 5 ps shows a red-shift of 20 nm, relative to the peak of BR. Its absorption remains constant for the observation time of 300 ps. (d) Upon suspending bacteriorhodopsin in D2O and deuterating the retinal Schiff base at its nitrogen (lysine 216), the same photoproducts J and K are observed. The relaxation time constants τ1 and τ2 remain unchanged upon deuteration within the experimental accuracy of 20%.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfano R. R., Govindjee R., Becher B., Ebrey T. G. Picosecond kinetics of the fluorescence from the chromophore of the purple membrane protein of Halobacterium halobium. Biophys J. 1976 May;16(5):541–545. doi: 10.1016/S0006-3495(76)85709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury M. L., Peters K. S., Rentzepis P. M. Primary intermediates in the photochemical cycle of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):375–382. doi: 10.1016/S0006-3495(78)85456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R., Cooper T. M. Energy storage in the primary step of the photocycle of bacteriorhodopsin. Biophys J. 1983 Apr;42(1):61–69. doi: 10.1016/S0006-3495(83)84369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio R., Gutowitz H., Mowery P., Taylor M., Stoeckenius W. Light-dark adaptation of bacteriorhodopsin in triton-treated purple membrane. Biochim Biophys Acta. 1980 Mar 7;590(1):13–23. doi: 10.1016/0005-2728(80)90142-5. [DOI] [PubMed] [Google Scholar]
- Chu Kung M., DeVault D., Hess B., Oesterhelt D. Photolysis of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):907–911. doi: 10.1016/S0006-3495(75)85864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Ebrey T. G., Crofts A. R. The quantum efficiency of proton pumping by the purple membrane of Halobacterium halobium. Biophys J. 1980 May;30(2):231–242. doi: 10.1016/S0006-3495(80)85091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. D., Marcus M. A., Lewis A., Mahr H., Frigo N. A method for measuring picosecond phenomena in photolabile species: the emission lifetime of bacteriorhodopsin. Biophys J. 1976 Dec;16(12):1399–1409. doi: 10.1016/S0006-3495(76)85783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen E. P., Shank C. V., Lewis A., Marcus M. A. Subpicosecond spectroscopy of bacteriorhodopsin. Science. 1978 Jun 16;200(4347):1279–1281. doi: 10.1126/science.663607. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Sundstrom V., Yamane T., Rentzepis P. M. Kinetics of the 580-nm ultrafast bacteriorhodopsin transient. Biophys J. 1978 Apr;22(1):121–124. doi: 10.1016/S0006-3495(78)85476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J. P., Perreault G. J. Observation of light emission from a rhodopsin. Nature. 1976 Apr 22;260(5553):675–678. doi: 10.1038/260675a0. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Kinetic resonance Raman spectroscopy: dynamics of deprotonation of the Schiff base of bacteriorhodopsin. Science. 1977 Mar 25;195(4284):1328–1330. doi: 10.1126/science.841330. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L. Reconstitution of bacteriorhodopsin. FEBS Lett. 1974 Aug 30;44(3):262–265. doi: 10.1016/0014-5793(74)81153-1. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. L., Campillo A. J., Lewis A., Perreault G. J., Spoonhower J. P., Clayton R. K., Stoeckenius W. Picosecond and steady state, variable intensity and variable temperature emission spectroscopy of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):383–393. doi: 10.1016/S0006-3495(78)85457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]
- Tavan P., Schulten K., Oesterhelt D. The effect of protonation and electrical interactions on the stereochemistry of retinal schiff bases. Biophys J. 1985 Mar;47(3):415–430. doi: 10.1016/S0006-3495(85)83933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner J., Hsieh C. L., Burns A. R., El-Sayed M. A. Time-resolved resonance Raman spectroscopy of intermediates of bacteriorhodopsin: The bK(590) intermediate. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3046–3050. doi: 10.1073/pnas.76.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]



