Abstract
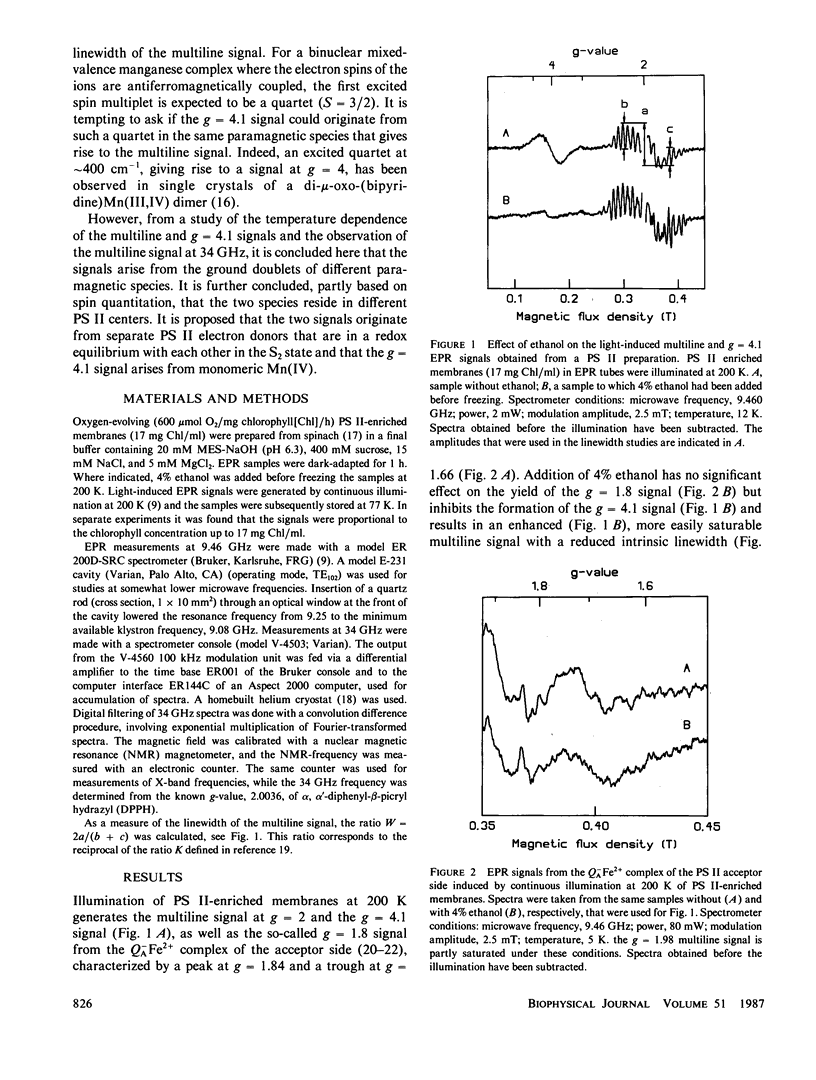
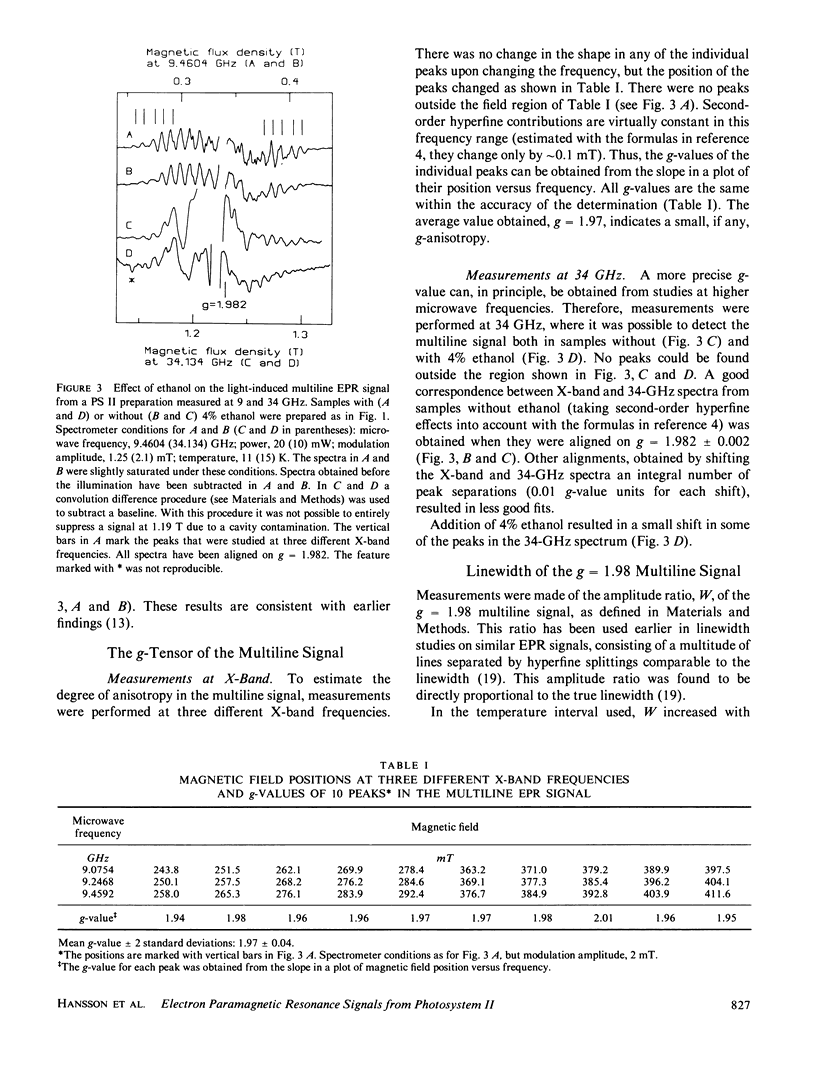
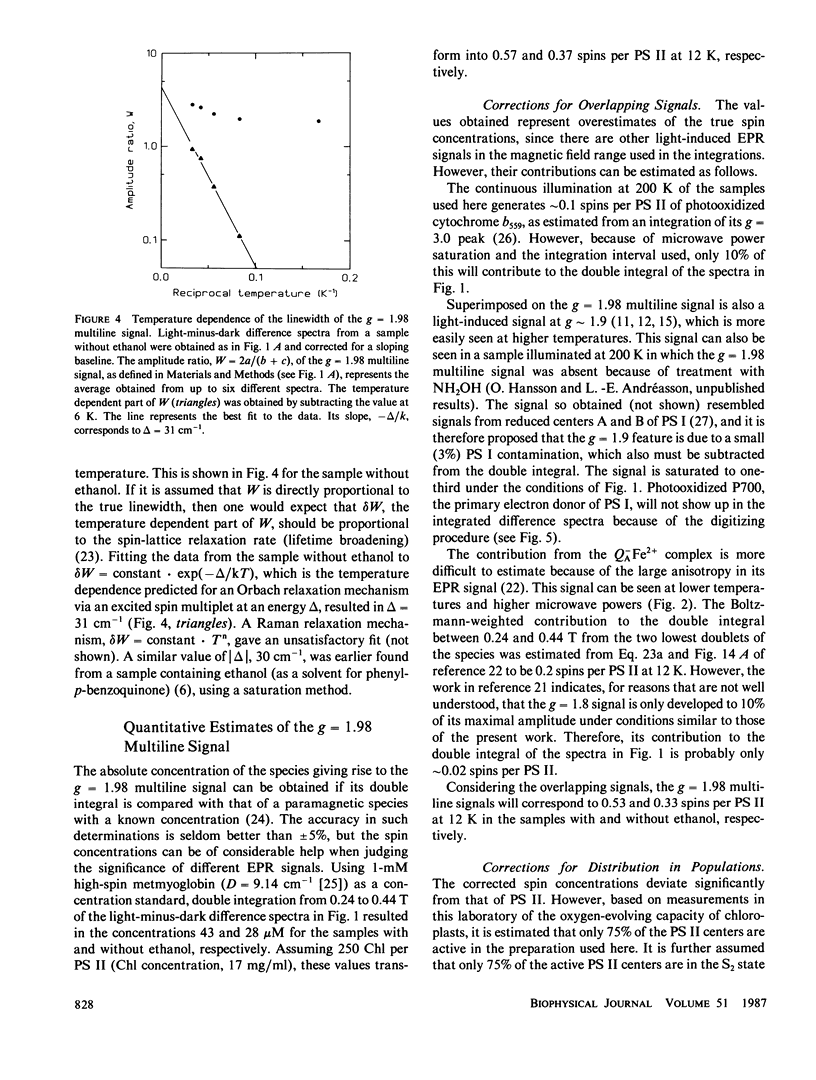
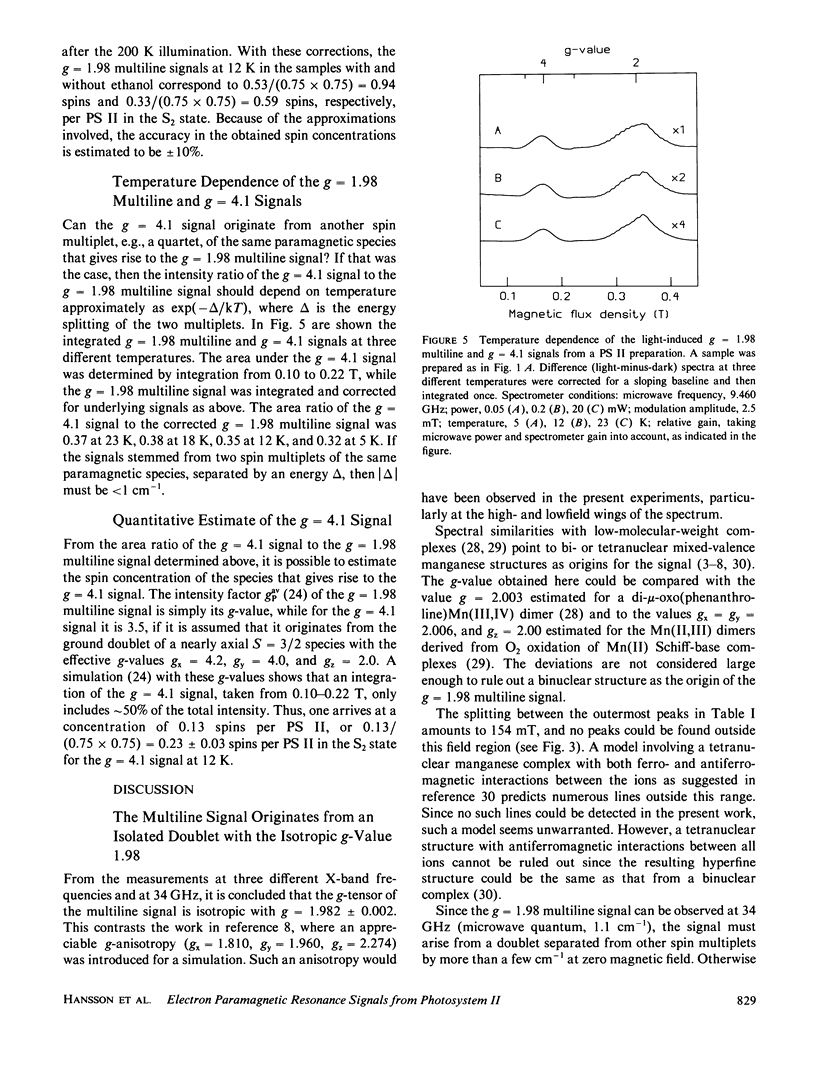
Continuous illumination at 200 K of photosystem (PS) II-enriched membranes generates two electron paramagnetic resonance (EPR) signals that both are connected with the S2 state: a multiline signal at g 2 and a single line at g = 4.1. From measurements at three different X-band frequencies and at 34 GHz, the g tensor of the multiline species was found to be isotropic with g = 1.982. It has an excited spin multiplet at ∼30 cm-1, inferred from the temperature-dependence of the linewidth. The intensity ratio of the g = 4.1 signal to the multiline signal was found to be almost constant from 5 to 23 K. Based on these findings and on spin quantitation of the two signals in samples with and without 4% ethanol, it is concluded that they arise from the ground doublets of paramagnetic species in different PS II centers. It is suggested that the two signals originate from separate PS II electron donors that are in a redox equilibrium with each other in the S2 state and that the g = 4.1 signal arises from monomeric Mn(IV).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardwood P., Gibson J. F., Bertrand P., Gayda J. P. Temperature dependence of the electronic spin-lattice relaxation time in a 2-iron-2-sulphur model complex. Biochim Biophys Acta. 1983 Jan 26;742(2):426–433. doi: 10.1016/0167-4838(83)90330-8. [DOI] [PubMed] [Google Scholar]
- Butler W. F., Calvo R., Fredkin D. R., Isaacson R. A., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. III. EPR measurements of the reduced acceptor complex. Biophys J. 1984 May;45(5):947–973. doi: 10.1016/S0006-3495(84)84241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes G. C., Siderer Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc Natl Acad Sci U S A. 1981 Jan;78(1):274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes C. P., Isaacson R. A., Feher G. Determination of the zero-field splitting of Fe 3+ in heme proteins from the temperature dependence of the spin-lattice relaxation rate. Biochim Biophys Acta. 1971 Jul 20;244(1):206–210. doi: 10.1016/0304-4165(71)90138-3. [DOI] [PubMed] [Google Scholar]
- de Paula J. C., Innes J. B., Brudvig G. W. Electron transfer in photosystem II at cryogenic temperatures. Biochemistry. 1985 Dec 31;24(27):8114–8120. doi: 10.1021/bi00348a042. [DOI] [PubMed] [Google Scholar]


