Abstract
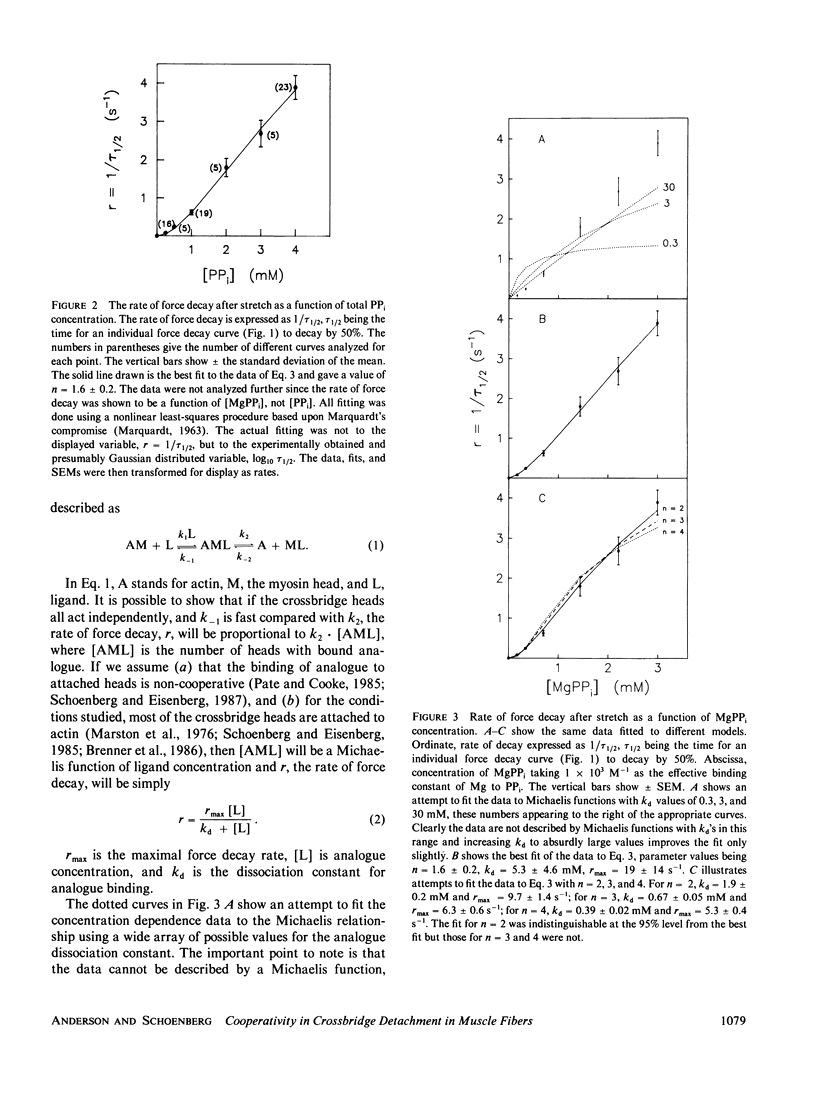
When rabbit psoas muscle fibers bathed in solutions containing the ATP analogue magnesium pyrophosphate (MgPPi) are first stretched rapidly and then held isometric, a force is generated during the stretch which decays during the subsequent isometric period (Schoenberg, M., and E. Eisenberg. 1985. Biophys. J. 48:863-871). Previously we showed that the force decay is due to crossbridge heads detaching and reattaching in positions of lesser strain, the rate of decay of force reflecting the crossbridge detachment rate constants. Since the crossbridge detachment rate constants with MgPPi bound to the active site are so much faster than without analogue bound, at subsaturating concentrations of analogue, if the heads act independently and nucleotide association and dissociation is rapid, the rate of force decay should simply be proportional to the number of heads with bound analogue. That is, the analogue concentration dependence of the rate of force decay should have the same form as the Michaelis-Menten equation. Here we report that the concentration dependence of the rate of force decay is not described by the Michaelis equation, but is instead sigmoidal. This suggests possible cooperativity in the detachment of the crossbridge heads, the amount of cooperativity being described by an interaction coefficient of approximately 2. One idea put forward to explain the data is that both of the heads of a crossbridge may need to bind analogue before the crossbridge can relax a substantial fraction of the tension it supports.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B., Chalovich J. M., Greene L. E., Eisenberg E., Schoenberg M. Stiffness of skinned rabbit psoas fibers in MgATP and MgPPi solution. Biophys J. 1986 Oct;50(4):685–691. doi: 10.1016/S0006-3495(86)83509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Greene L. E., Eisenberg E., Schoenberg M. Ca2+-sensitive cross-bridge dissociation in the presence of magnesium pyrophosphate in skinned rabbit psoas fibers. Biophys J. 1986 Dec;50(6):1101–1108. doi: 10.1016/S0006-3495(86)83554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaen S., Shimada M., Sugi H. Evidence for cooperative interactions of myosin heads with thin filament in the force generation of vertebrate skeletal muscle fibers. J Biol Chem. 1986 Oct 15;261(29):13632–13636. [PubMed] [Google Scholar]
- Clarke M. L., Tregear R. T. Tension maintenance and crossbridge detachment. FEBS Lett. 1982 Jul 5;143(2):217–219. doi: 10.1016/0014-5793(82)80102-6. [DOI] [PubMed] [Google Scholar]
- Frey C. M., Stuehr J. E. Interactions of divalent metal ions with inorganic and nucleoside phosphates. I. Thermodynamics. J Am Chem Soc. 1972 Dec 13;94(25):8898–8904. doi: 10.1021/ja00780a042. [DOI] [PubMed] [Google Scholar]
- Hackney D. D., Clark P. K. Catalytic consequences of oligomeric organization: kinetic evidence for "tethered" acto-heavy meromyosin at low ATP concentrations. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5345–5349. doi: 10.1073/pnas.81.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad M., Goody R. S. Kinetic and thermodynamic properties of the ternary complex between F-actin, myosin subfragment 1 and adenosine 5'-[beta, gamma-imido]triphosphate. Eur J Biochem. 1982 Nov 15;128(2-3):547–555. doi: 10.1111/j.1432-1033.1982.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H. J. Cross bridge slippage induced by the ATP analogue AMP-PNP and stretch in glycerol-extracted fibrillar muscle fibres. Biophys Struct Mech. 1978 Apr 13;4(2):159–168. doi: 10.1007/BF00539229. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Rodger C. D., Tregear R. T. Changes in muscle crossbridges when beta, gamma-imido-ATP binds to myosin. J Mol Biol. 1976 Jun 14;104(1):263–276. doi: 10.1016/0022-2836(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. The inhibition of muscle contraction by adenosine 5' (beta, gamma-imido) triphosphate and by pyrophosphate. Biophys J. 1985 Jun;47(6):773–780. doi: 10.1016/S0006-3495(85)83980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Brenner B., Chalovich J. M., Greene L. E., Eisenberg E. Cross-bridge attachment in relaxed muscle. Adv Exp Med Biol. 1984;170:269–284. doi: 10.1007/978-1-4684-4703-3_24. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. ADP binding to myosin cross-bridges and its effect on the cross-bridge detachment rate constants. J Gen Physiol. 1987 Jun;89(6):905–920. doi: 10.1085/jgp.89.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys J. 1985 Dec;48(6):863–871. doi: 10.1016/S0006-3495(85)83847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Equilibrium muscle cross-bridge behavior. Theoretical considerations. Biophys J. 1985 Sep;48(3):467–475. doi: 10.1016/S0006-3495(85)83802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep J., Glyn H. Inhibition of myofibrillar and actomyosin subfragment 1 adenosinetriphosphatase by adenosine 5'-diphosphate, pyrophosphate, and adenyl-5'-yl imidodiphosphate. Biochemistry. 1986 Mar 11;25(5):1149–1154. doi: 10.1021/bi00353a030. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Tözeren A., Schoenberg M. The effect of cross-bridge clustering and head-head competition on the mechanical response of skeletal muscle under equilibrium conditions. Biophys J. 1986 Nov;50(5):875–884. doi: 10.1016/S0006-3495(86)83528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



