Abstract
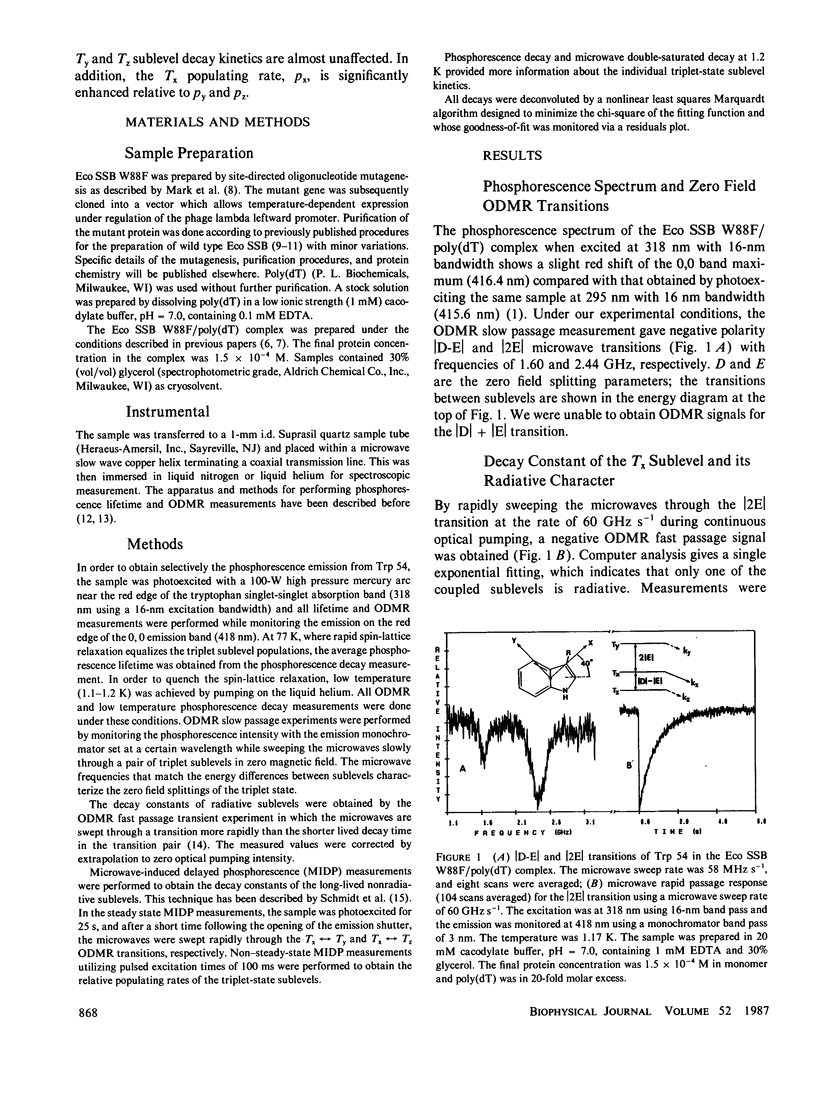
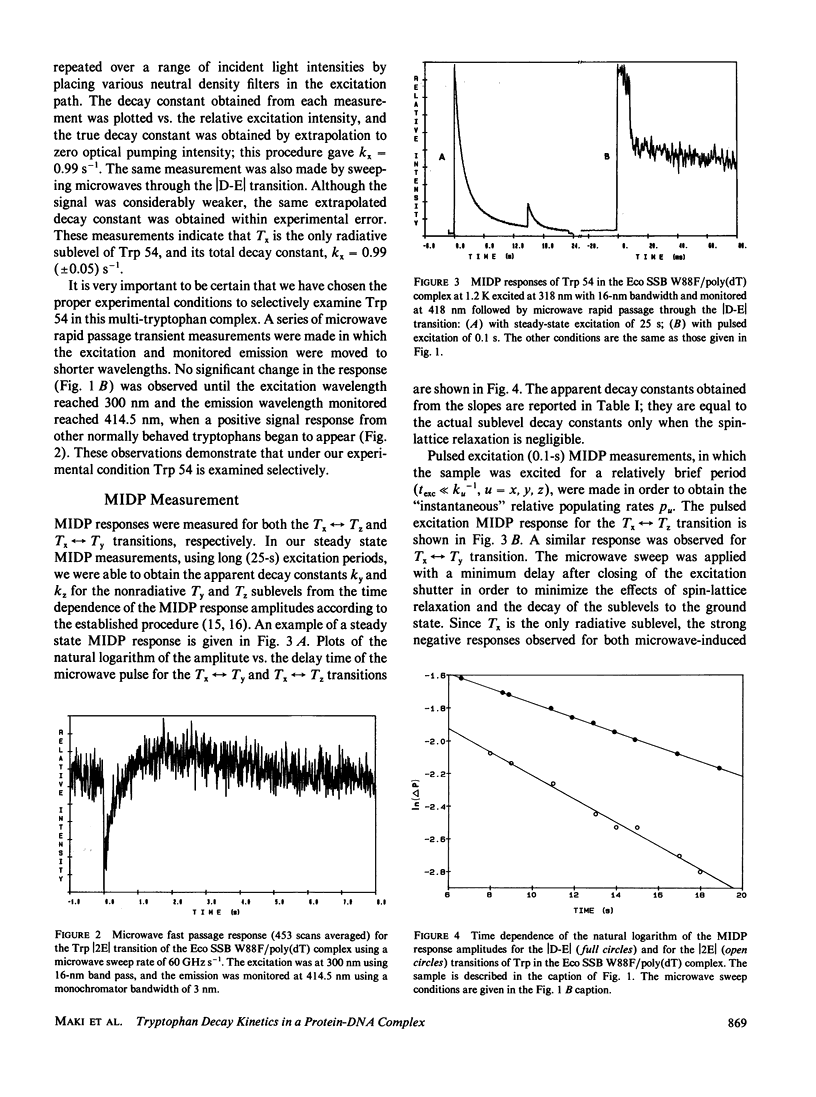
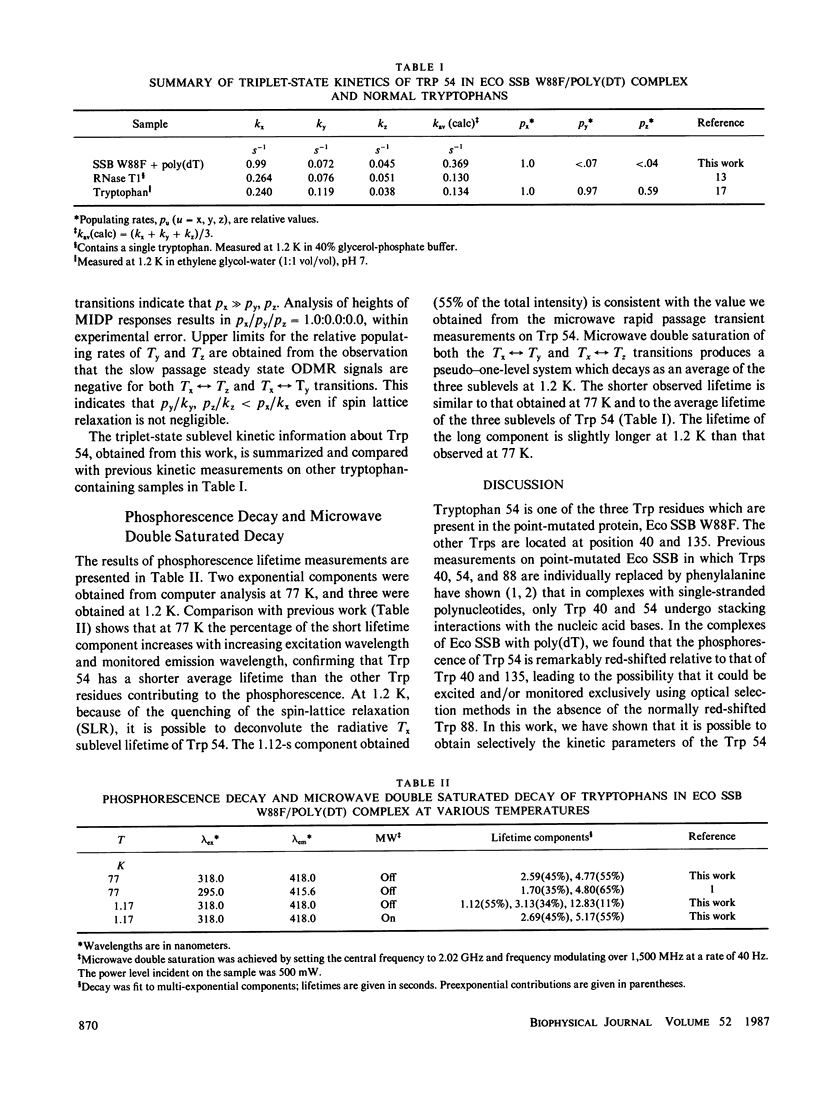
The individual sublevel kinetics of the lowest triplet state of tryptophan 54 (Trp 54) which is highly perturbed in the complex of Escherichia coli single-stranded DNA binding protein (Eco SSB) with poly(deoxythymidylic) acid (poly[dT]) have been studied by optically detected magnetic resonance (ODMR) spectroscopy. The triplet sublevel decay constants of Trp 54, kx, ky, kz, are 0.99, 0.072, and 0.045 s-1, respectively, in the poly(dT) complex of a point-mutated Eco SSB in which Trp 88 is substituted by phenylalanine. Tx is the only radiative triplet sublevel. Negative polarity of the Tx----Tz and Tx----Ty phosphorescence-detected ODMR signals results from the steady state population pattern, nx greater than ny, nz, and implies that the relations, px greater than or equal to 14py, and px greater than or equal to 22pz exist for the relative populating rates. Spin-orbit coupling between radiative singlet states and the Tx sublevel of the lowest triplet state of Trp 54 is enhanced selectively upon complexing of Eco SSB with poly(dT).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cha T. A., Maki A. H. Close range interactions between nucleotide bases and tryptophan residues in an Escherichia coli single-stranded DNA binding protein-mercurated poly(uridylic acid) complex. A study by optically detected magnetic resonance spectroscopy. J Biol Chem. 1984 Jan 25;259(2):1105–1109. [PubMed] [Google Scholar]
- Chase J. W., L'Italien J. J., Murphy J. B., Spicer E. K., Williams K. R. Characterization of the Escherichia coli SSB-113 mutant single-stranded DNA-binding protein. Cloning of the gene, DNA and protein sequence analysis, high pressure liquid chromatography peptide mapping, and DNA-binding studies. J Biol Chem. 1984 Jan 25;259(2):805–814. [PubMed] [Google Scholar]
- Chase J. W., Whittier R. F., Auerbach J., Sancar A., Rupp W. D. Amplification of single-strand DNA binding protein in Escherichia coli. Nucleic Acids Res. 1980 Jul 25;8(14):3215–3227. doi: 10.1093/nar/8.14.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Post-tetanic depolarization in sympathetic neurones of the guinea-pig. J Physiol. 1982 Feb;323:325–337. doi: 10.1113/jphysiol.1982.sp014075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Petrin M., Maki A. Spin-lattice relaxation in the triplet state of the buried tryptophan residue of ribonuclease T1. Biophys J. 1986 Mar;49(3):753–760. doi: 10.1016/S0006-3495(86)83701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamis M. I., Casas-Finet J. R., Maki A. H., Murphy J. B., Chase J. W. Investigation of the role of individual tryptophan residues in the binding of Escherichia coli single-stranded DNA binding protein to single-stranded polynucleotides. A study by optical detection of magnetic resonance and site-selected mutagenesis. J Biol Chem. 1987 Aug 15;262(23):10938–10945. [PubMed] [Google Scholar]
- Khamis M. I., Casas-Finet J. R., Maki A. H., Murphy J. B., Chase J. W. Role of tryptophan 54 in the binding of E. coli single-stranded DNA-binding protein to single-stranded polynucleotides. FEBS Lett. 1987 Jan 26;211(2):155–159. doi: 10.1016/0014-5793(87)81427-8. [DOI] [PubMed] [Google Scholar]
- Khamis M. I., Casas-Finet J. R., Maki A. H., Ruvolo P. P., Chase J. W. Optically detected magnetic resonance of tryptophan residues in complexes formed between a bacterial single-stranded DNA binding protein and heavy atom modified poly(uridylic acid). Biochemistry. 1987 Jun 16;26(12):3347–3354. doi: 10.1021/bi00386a015. [DOI] [PubMed] [Google Scholar]
- Khamis M. I., Casas-Finet J. R., Maki A. H. Stacking interactions of tryptophan residues and nucleotide bases in complexes formed between Escherichia coli single-stranded DNA binding protein and heavy atom-modified poly(uridylic) acid. A study by optically detected magnetic resonance spectroscopy. J Biol Chem. 1987 Feb 5;262(4):1725–1733. [PubMed] [Google Scholar]
- Khamis M. I., Maki A. H. Investigation of complexes formed between gene 32 protein from bacteriophage T4 and heavy-atom-modified single-stranded polynucleotides using optical detection of magnetic resonance. Biochemistry. 1986 Oct 7;25(20):5865–5872. doi: 10.1021/bi00368a005. [DOI] [PubMed] [Google Scholar]
- Maki A. H., Co T. Study of triple-singlet energy transfer in an enzyme-dye complex using optical detection of magnetic resonance. Biochemistry. 1976 Mar 23;15(6):1229–1235. doi: 10.1021/bi00651a009. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Lu S. D., Creasey A. A., Yamamoto R., Lin L. S. Site-specific mutagenesis of the human fibroblast interferon gene. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5662–5666. doi: 10.1073/pnas.81.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]


