Abstract
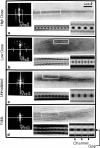
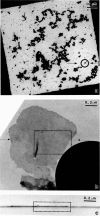
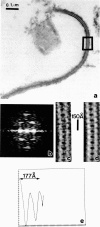
Profiles of negatively stained gap junctions have been measured by grid sectioning. After normal levels of electron irradiation, the membrane thickness shrinks to about half that of unirradiated controls, but no shrinkage occurs in the hexagonal lattice plane. Even under low irradiation conditions, there is significant thinning of the membranes. Edge views, in which rows of connexons are aligned parallel to the beam, were obtained from grid sections, folds in normal negatively stained specimens, and sections of a positively stained specimen. Averaging these micrographs with the translational and mirror symmetry of the projected lattice image displays conserved and variable features in the stain distribution of different specimens. Variations in the relative amount of negative stain in the gap at the surfaces and in the channel are uncorrelated with the irradiation but appear to depend on the local staining conditions and the integrity of the connexons. The dimensions measured from previously unirradiated grid sections, folds, and positively stained sections are in accord with x-ray diffraction measurements. Radiation-induced shrinkage can be accounted for by mass loss principally from the membrane bilayer. Disordering of the surface structure appears to be correlated with the radiation sensitivity of the bilayer; in contrast, the gap structure is well preserved under a variety of conditions.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Amos L. A. Structure of the tubulin dimer in zinc-induced sheets. J Mol Biol. 1978 Jul 25;123(1):89–106. doi: 10.1016/0022-2836(78)90378-9. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Hollingshead C. J., Goodenough D. A. Gap junction structures. IV. Asymmetric features revealed by low-irradiation microscopy. J Cell Biol. 1983 Jan;96(1):204–216. doi: 10.1083/jcb.96.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Sosinsky G. E., Caspar D. L., Gall C., Goodenough D. A. Gap junction structures. VII. Analysis of connexon images obtained with cationic and anionic negative stains. J Mol Biol. 1985 Jul 5;184(1):81–98. doi: 10.1016/0022-2836(85)90045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Berriman J., Leonard K. R. Methods for specimen thickness determination in electron microscopy. II. Changes in thickness with dose. Ultramicroscopy. 1986;19(4):349–366. doi: 10.1016/0304-3991(86)90095-1. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Goodenough D. A., Makowski L., Phillips W. C. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977 Aug;74(2):605–628. doi: 10.1083/jcb.74.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. F., Goodenough D. A. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981 Aug;90(2):521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R., Leonard K. R. Comparative mass measurement of biological macromolecules by scanning transmission electron microscopy. J Microsc. 1981 Jun;122(Pt 3):275–286. doi: 10.1111/j.1365-2818.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M. Limitations to significant information in biological electron microscopy as a result of radiation damage. J Ultrastruct Res. 1971 Aug;36(3):466–482. doi: 10.1016/s0022-5320(71)80118-1. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. In vitro formation of gap junction vesicles. J Cell Biol. 1976 Feb;68(2):220–231. doi: 10.1083/jcb.68.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Heuser J. The inside and outside of gap-junction membranes visualized by deep etching. Cell. 1982 Sep;30(2):395–406. doi: 10.1016/0092-8674(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Jésior J. C. A new approach for the visualization of molecular arrangement in biological micro-crystals. Ultramicroscopy. 1982;8(4):379–384. doi: 10.1016/0304-3991(82)90060-2. [DOI] [PubMed] [Google Scholar]
- Jésior J. C., Bois J. M. Dispositif de repérage micrométrique in situ d'un objet sur une grille de microscope. Application à l'observation des coupes sériées. Biol Cell. 1984;51(3):399–402. doi: 10.1111/j.1768-322x.1984.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Crowther R. A. Three-dimensional reconstruction from tilted sections of fish muscle M-band. Nature. 1984 Feb 9;307(5951):566–568. doi: 10.1038/307566a0. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Goodenough D. A., Phillips W. C. Gap Junction Structures: III. The Effect of Variations in the Isolation Procedure. Biophys J. 1982 Jan;37(1):189–191. doi: 10.1016/S0006-3495(82)84663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. V. Structural chemistry inferred from X-ray diffraction measurements on sucrose accessibility and trypsin susceptibility. J Mol Biol. 1984 Apr 15;174(3):449–481. doi: 10.1016/0022-2836(84)90331-0. [DOI] [PubMed] [Google Scholar]
- Stenn K., Bahr G. F. Specimen damage caused by the beam of the transmission electron microscope, a correlative reconsideration. J Ultrastruct Res. 1970 Jun;31(5-6):526–550. doi: 10.1016/s0022-5320(70)90167-x. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Cordova L., Reedy M. K. Image reconstruction using electron micrographs of insect flight muscle. Use of thick transverse sections to supplement data from tilted thin longitudinal sections. Biophys J. 1986 Jan;49(1):353–364. doi: 10.1016/S0006-3495(86)83648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N. Beef liver catalase structure: interpretation of electron micrographs. J Mol Biol. 1975 Oct 15;98(1):235–242. doi: 10.1016/s0022-2836(75)80111-2. [DOI] [PubMed] [Google Scholar]
- Unwin P. N. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. II. The influence of electron irradiation of the stain distribution. J Mol Biol. 1974 Aug 25;87(4):657–670. doi: 10.1016/0022-2836(74)90076-x. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Two configurations of a channel-forming membrane protein. Nature. 1984 Feb 16;307(5952):609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Zampighi G., Corless J. M., Robertson J. D. On gap junction structure. J Cell Biol. 1980 Jul;86(1):190–198. doi: 10.1083/jcb.86.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]