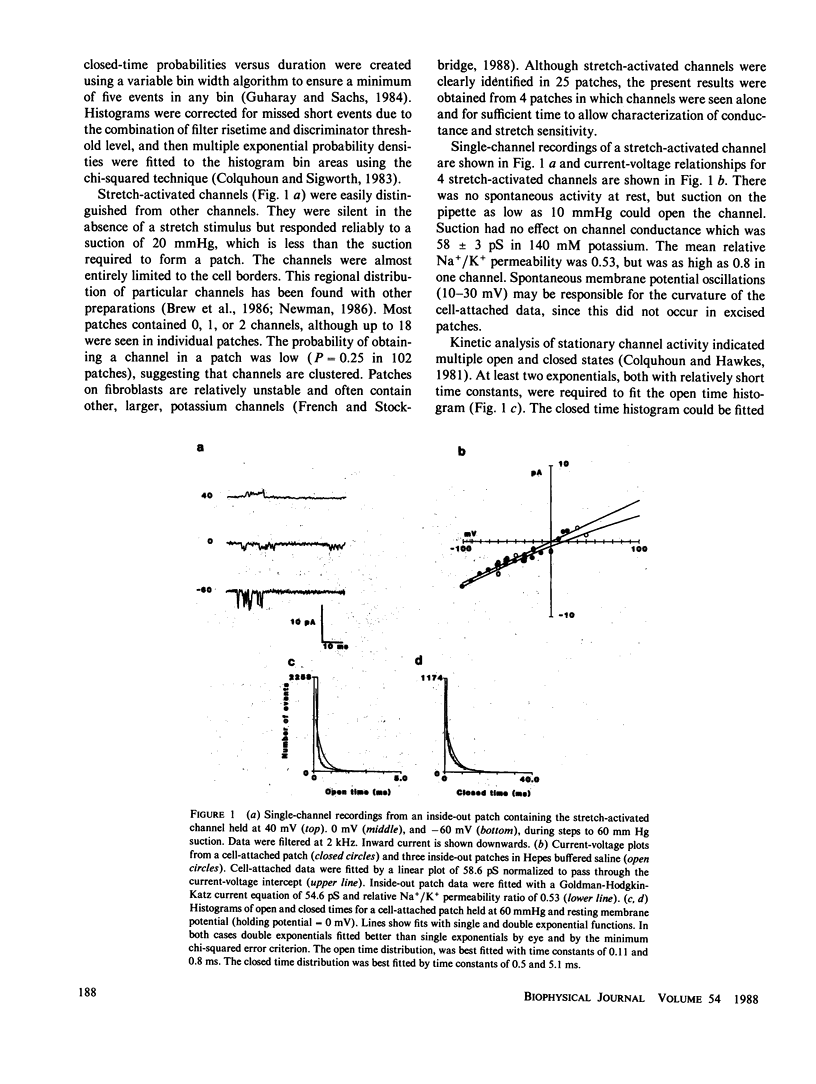
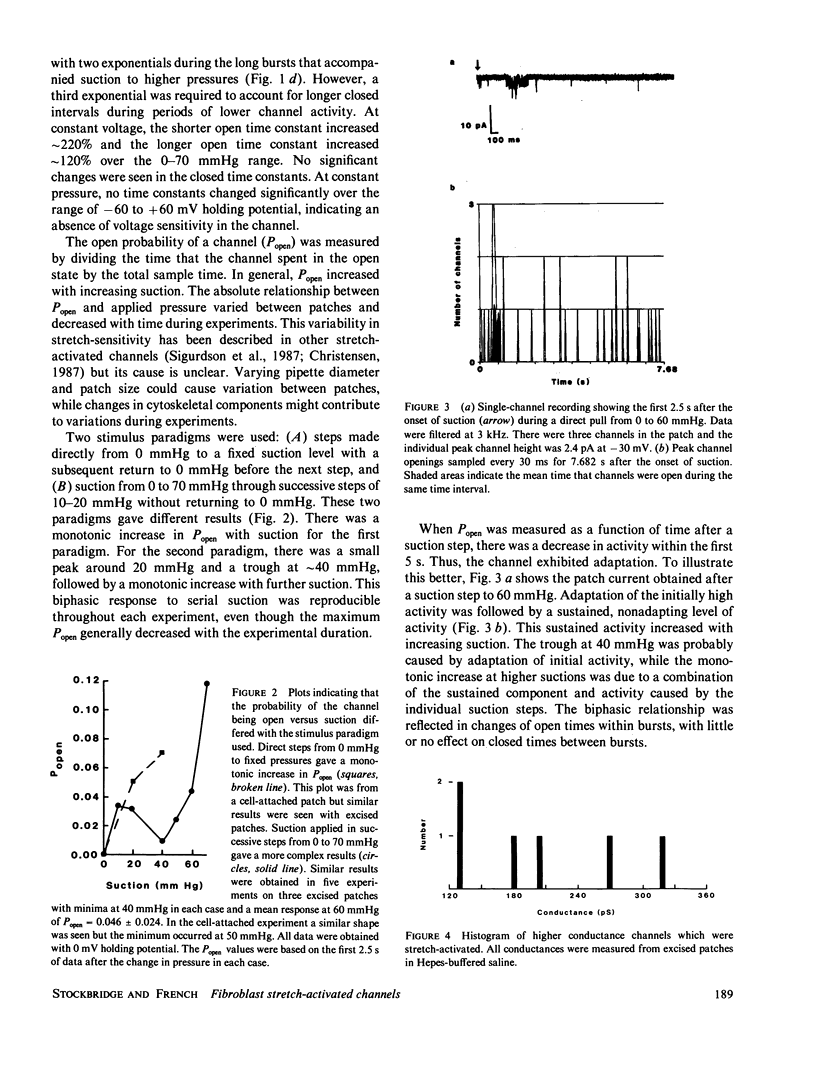
Abstract
Nonconfluent fibroblasts are relatively depolarized when compared with confluent fibroblasts, and transient hyperpolarizations result from a range of external stimuli as well as internal cellular activities. This electrical activity ceases, along with growth and mitogenic activity, when the cells become confluent. A calcium-activated potassium conductance is thought to be responsible for these hyperpolarizations, but in human fibroblasts the large calcium-activated potassium channel is not stretch-activated. We report here the identification of single stretch-activated cation channels in human fibroblasts, using the cell-attached and inside-out patch clamp techniques. The most prominent channel had a conductance of approximately 60 pS (picoSeimens) in 140 mM potassium and was permeable to potassium and sodium. The channel showed significant adaptation of activity when stretch was maintained over a period of several seconds, but a static component persisted for much longer periods. Higher conductance channels were also observed in a few excised patches.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binggeli R., Weinstein R. C. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986 Dec 21;123(4):377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Brew H., Gray P. T., Mobbs P., Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986 Dec 4;324(6096):466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- French A. S., Stockbridge L. L. Potassium channels in human and avian fibroblasts. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):395–412. doi: 10.1098/rspb.1988.0003. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi S., Slayman C. L. Membrane voltage, resistance, and channel switching in isolated mouse fibroblasts (L cells): a patch-electrode analysis. J Physiol. 1985 Oct;367:267–290. doi: 10.1113/jphysiol.1985.sp015824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J., Minna J. An active electrical response in fibroblasts. J Gen Physiol. 1972 Jul;60(1):58–71. doi: 10.1085/jgp.60.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A. High potassium conductance in astrocyte endfeet. Science. 1986 Jul 25;233(4762):453–454. doi: 10.1126/science.3726539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T., Yano J., Yawo H. Phagocytic activity and hyperpolarizing responses in L-strain mouse fibroblasts. J Physiol. 1981;313:101–119. doi: 10.1113/jphysiol.1981.sp013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Castro G. M. Ca2+-sensitive K+ channels in phagocytic cell membranes. Cell Calcium. 1983 Dec;4(5-6):475–492. doi: 10.1016/0143-4160(83)90023-4. [DOI] [PubMed] [Google Scholar]
- Tsuchiya W., Okada Y., Yano J., Murai A., Miyahara T., Tanaka T. Membrane potential changes associated with pinocytosis of serum lipoproteins in L cells. Exp Cell Res. 1981 Dec;136(2):271–278. doi: 10.1016/0014-4827(81)90005-7. [DOI] [PubMed] [Google Scholar]


