Abstract
1. The effect of glucose and of bovine γ-globulin on short-circuit current and sodium transport was measured using new-born pig ileum before and after suckling.
2. Glucose increased the short-circuit current with an apparent Km of 1·9 and 19·5 mM for new-born unsuckled and suckled intestines respectively. The Vmax was about 80 μA cm-2 in each case.
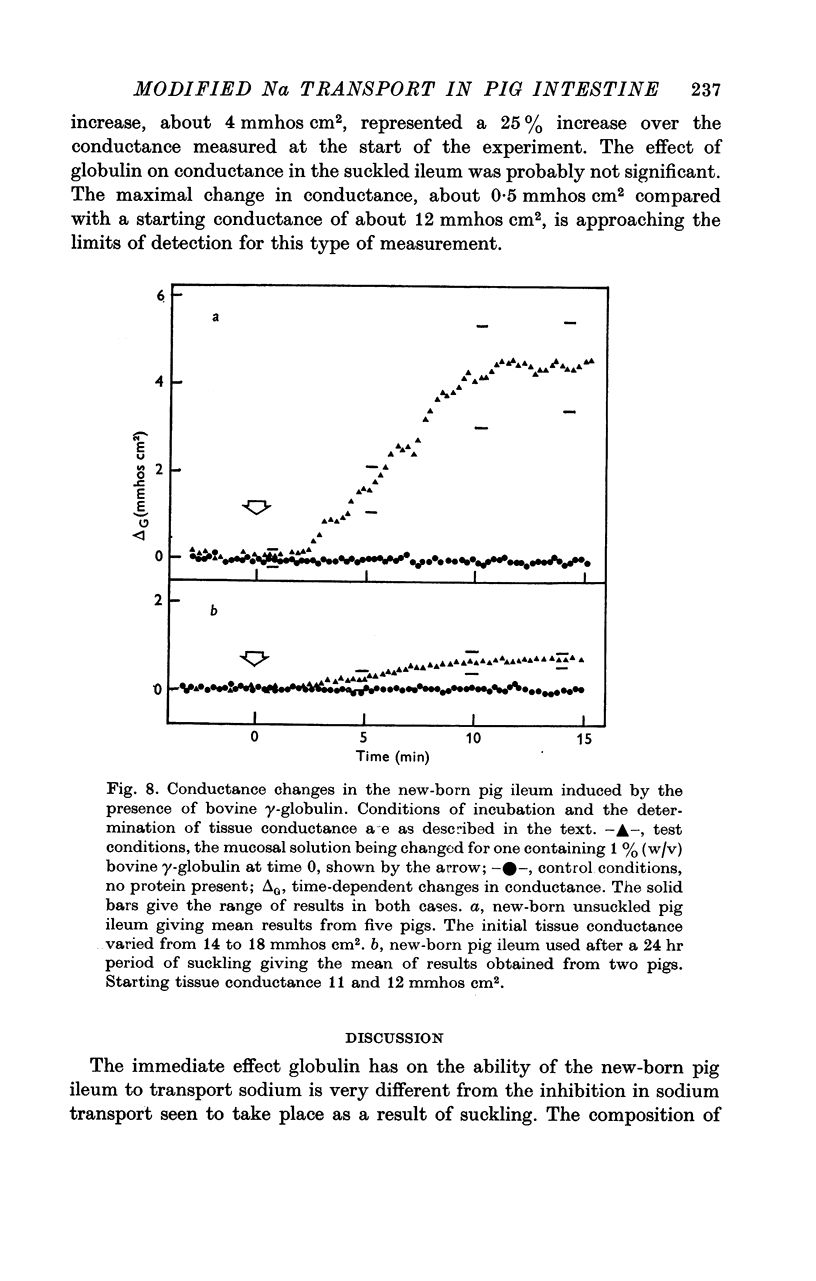
3. Bovine γ-globulin applied to new-born pig ileum caused a 20% increase in sodium chloride influx without change of short-circuit current. The tissue conductance was also greater in the presence of globulin. These effects were not seen in ileum taken from suckled pigs.
4. p-Chloromercuriphenyl sulphonic acid (PCMPS) inhibited the globulin induced increase in sodium influx in new-born pig ileum without changing the measured short-circuit current. 0·1 mM-PCMPS also changed the characteristics of the glucose-dependent increase in short-circuit current, so that this response came to resemble that seen in ileum taken from suckled pigs. Dithioerythritol reversed this effect of PCMPS.
5. The suckling-dependent difference in results is explained in terms of a change in microvillar function taking place as a consequence of long-term pinocytosis in vivo. The ability of PCMPS to simulate some of these changes suggests that sulphydryl groups might be important regulators of microvillar membrane stability.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baintner K., Jr The physiological role of colostral trypsin inhibitor: experiments with piglets and kittens. Acta Vet Acad Sci Hung. 1973 May;23(3):247–260. [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Cornell R., Walker W. A., Isselbacher K. J. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971 Jul;25(1):42–48. [PubMed] [Google Scholar]
- Hardy R. N., Hockaday A. R., Tapp R. L. Observations on the structure of the small intestine in foetal, neo-natal and suckling pigs. Philos Trans R Soc Lond B Biol Sci. 1971 Feb 11;259(834):517–531. doi: 10.1098/rstb.1971.0002. [DOI] [PubMed] [Google Scholar]
- Hardy R. N. The absorption of polyvinyl pyrrolidone by the new-born pig intestine. J Physiol. 1969 Oct;204(3):633–651. doi: 10.1113/jphysiol.1969.sp008936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. N. The break-down of 131-I-gamma-globulin in the digestive tract of the new-born pig. J Physiol. 1969 Nov;205(2):435–451. doi: 10.1113/jphysiol.1969.sp008976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques de Jesus C., Smith M. W. Sodium transport by the small intestine of new-born and suckling pigs. J Physiol. 1974 Nov;243(1):211–224. doi: 10.1113/jphysiol.1974.sp010750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn E. A., Smith M. W. Proceedings: Uptake of albumin by neonatal pig ileum incubated in vitro. J Physiol. 1974 Oct;242(2):30P–32P. [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Coupled sodium-chloride influx across the brush border of rabbit ileum. Am J Physiol. 1973 Aug;225(2):467–475. doi: 10.1152/ajplegacy.1973.225.2.467. [DOI] [PubMed] [Google Scholar]
- Pierce A. E., Smith M. W. The intestinal absorption of pig and bovine immune lactoglobulin and human serum albumin by the new-born pig. J Physiol. 1967 May;190(1):1–18. doi: 10.1113/jphysiol.1967.sp008189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J. F., Preston R. L., Curran P. F. Inhibition of amino acid transport in rabbit intestine by p-chloromercuriphenyl sulfonic acid. J Gen Physiol. 1973 Aug;62(2):131–146. doi: 10.1085/jgp.62.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]


