Abstract
1. A two-component inhibition, consisting of a rapid and slow i.p.s.p., has been observed in the medial cells of the pleural ganglion of Aplysia. Each i.p.s.p. has been shown to be mediated by a distinct cholinergic receptor. The ionic mechanisms of the two components of the inhibitory response (whether elicited synaptically or by ACh injection) are analysed in this paper.
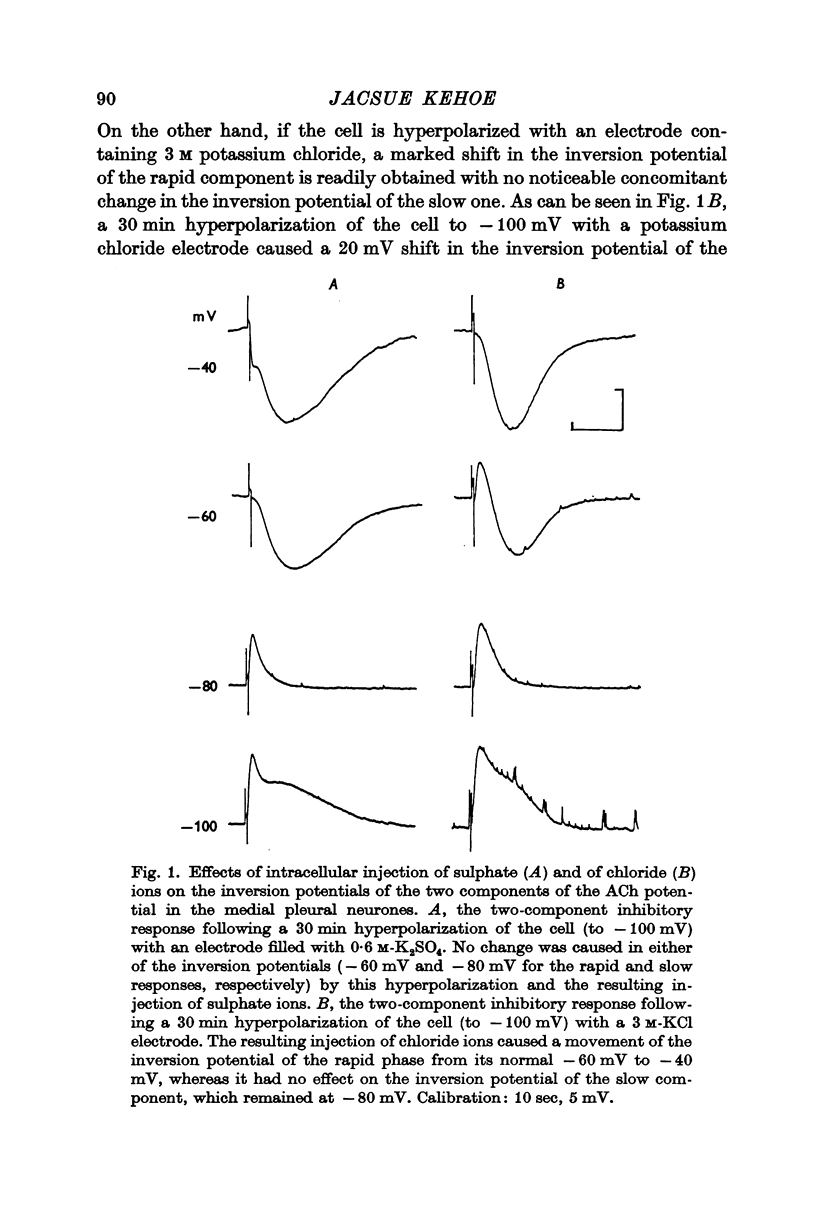
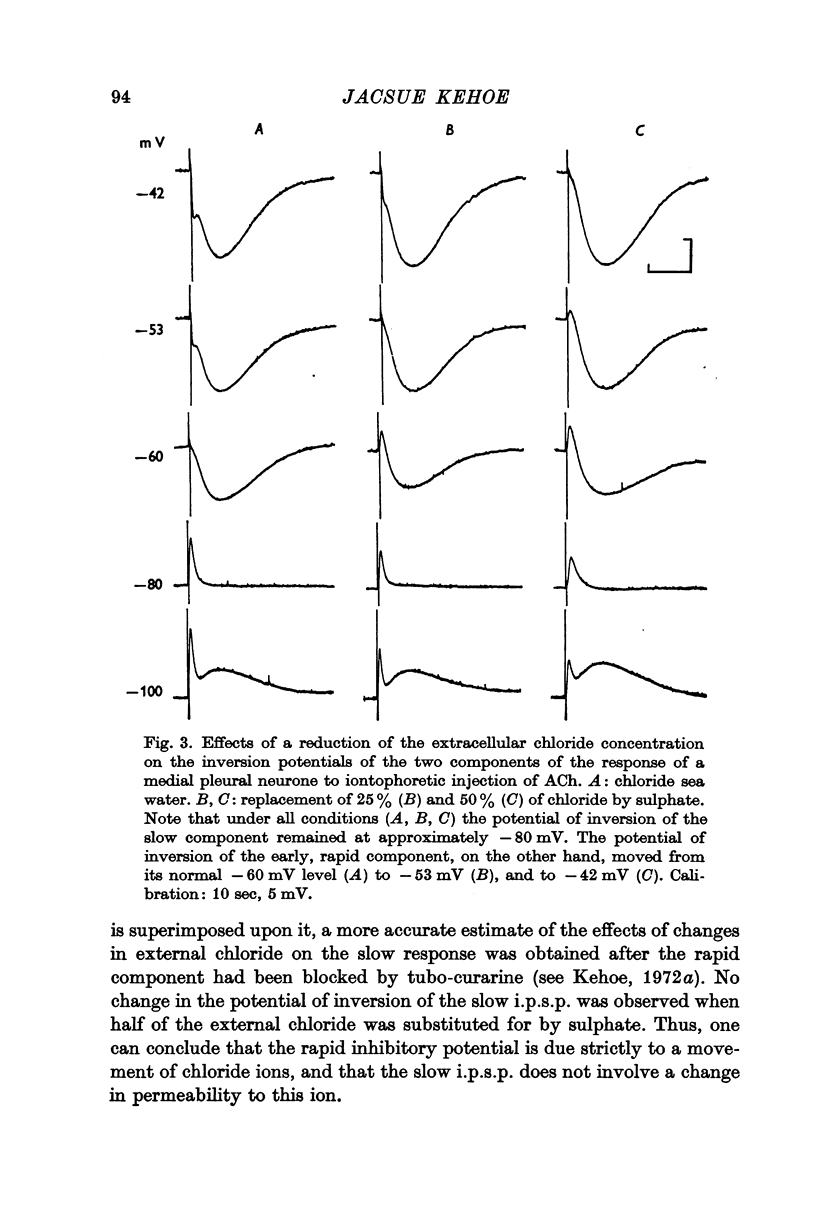
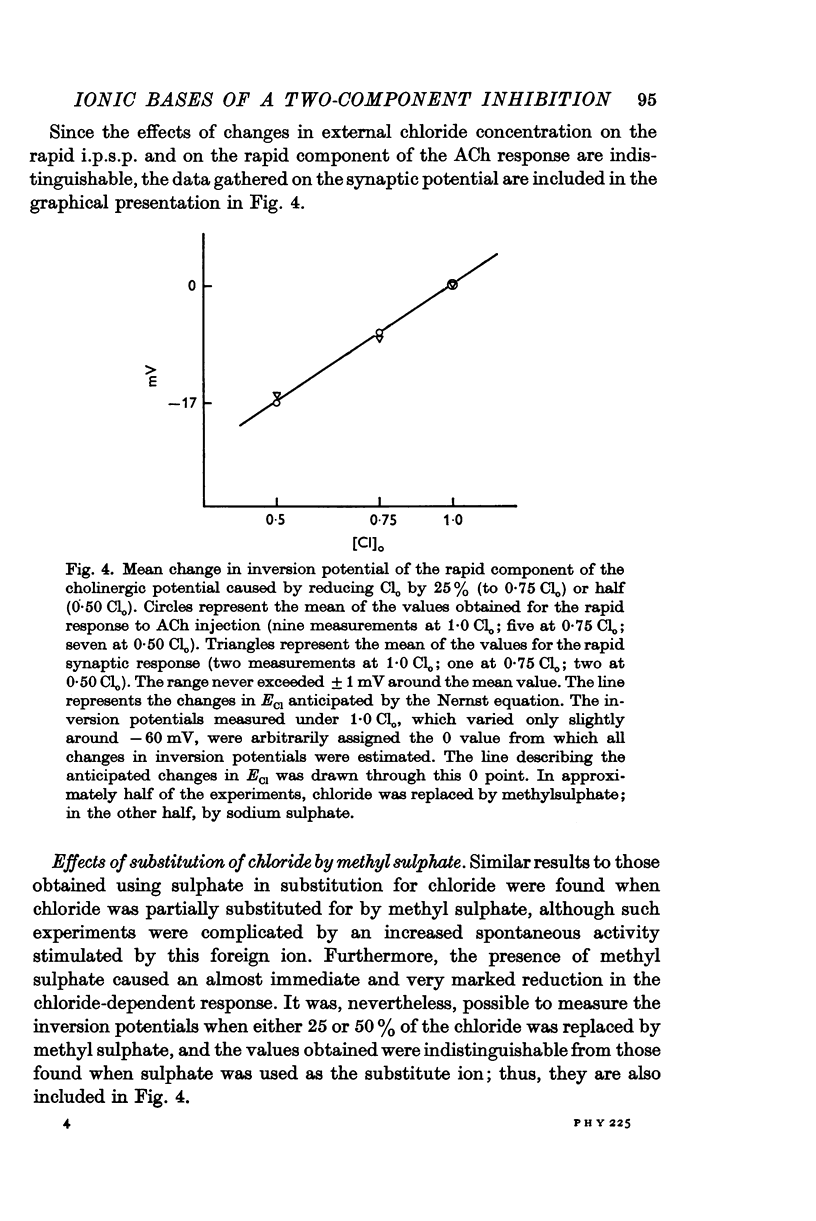
2. The inversion potential (typically -60 mV) of the rapid i.p.s.p. and of the rapid response to ACh injection is selectively altered by an intracellular injection of chloride or by partial substitution of the external chloride by impermeant anions. The shift caused by this last procedure is similar to that predicted for the chloride equilibrium potential (ECl) by the Nernst equation.
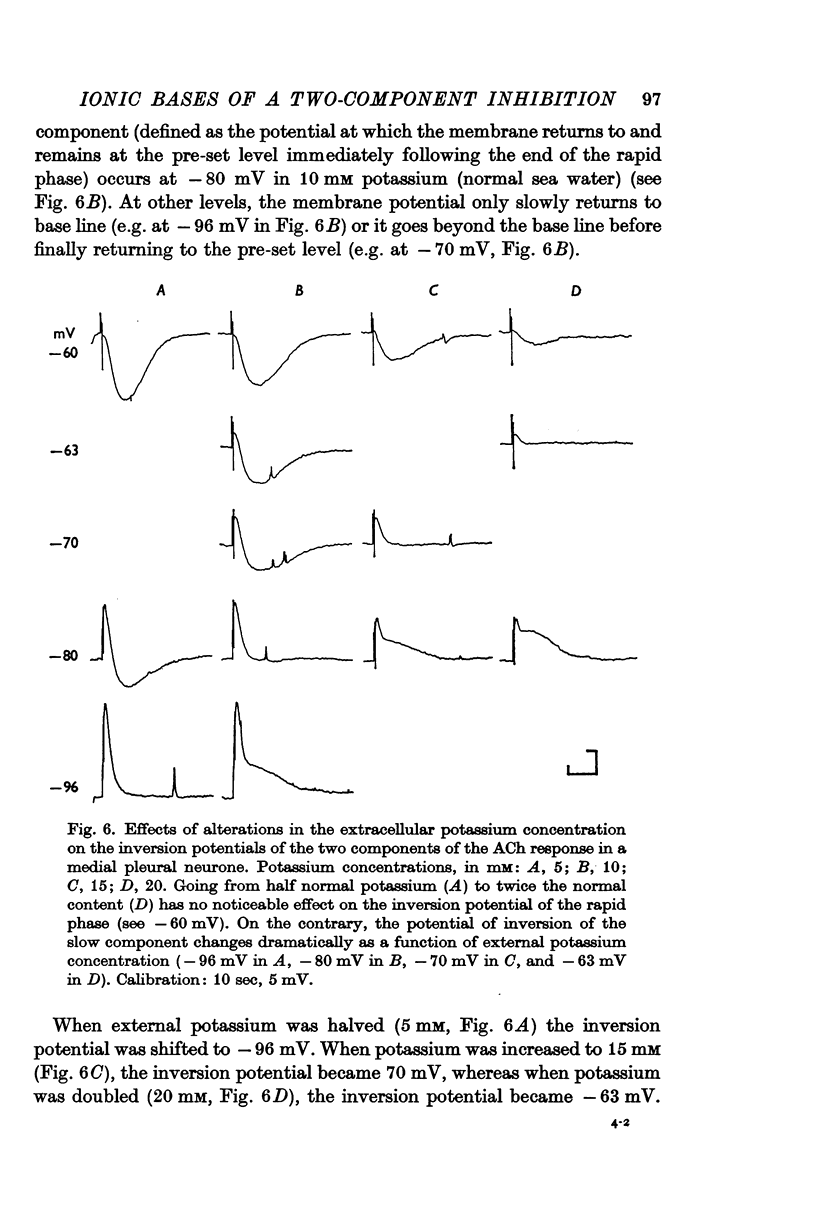
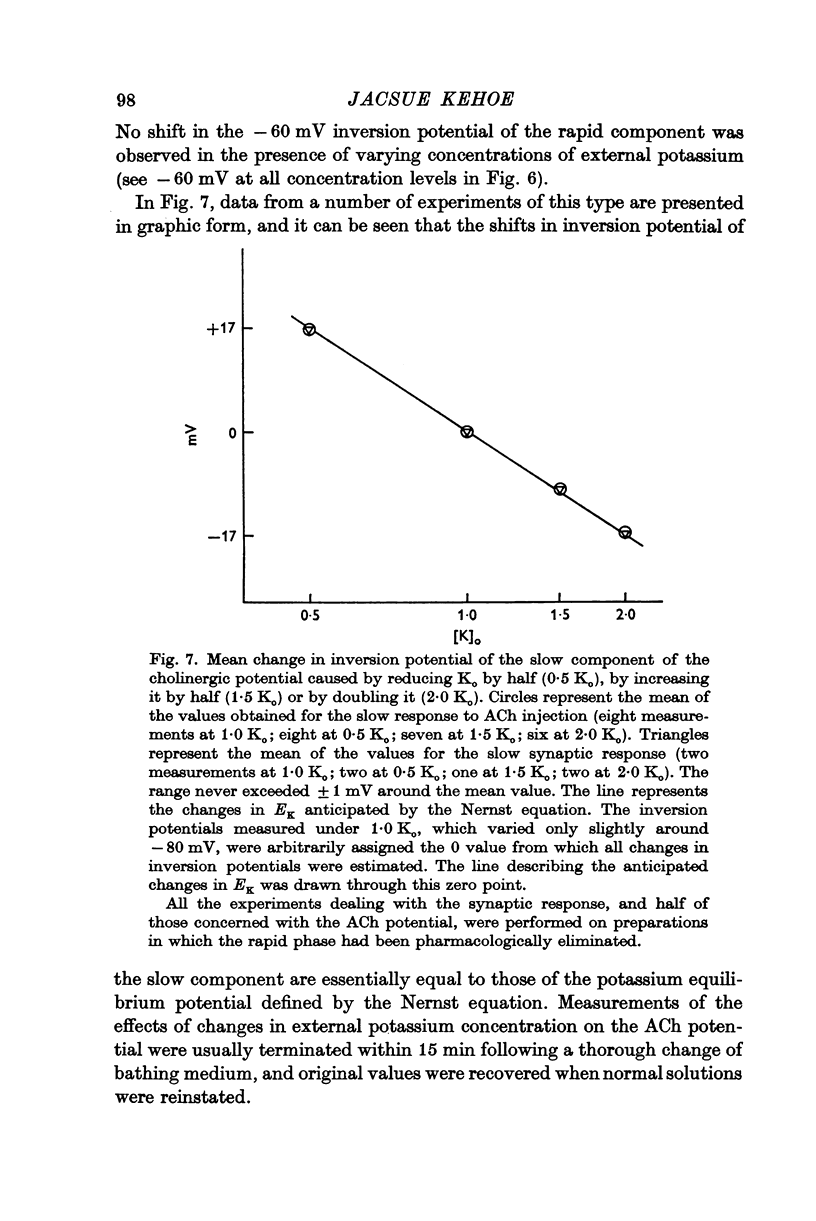
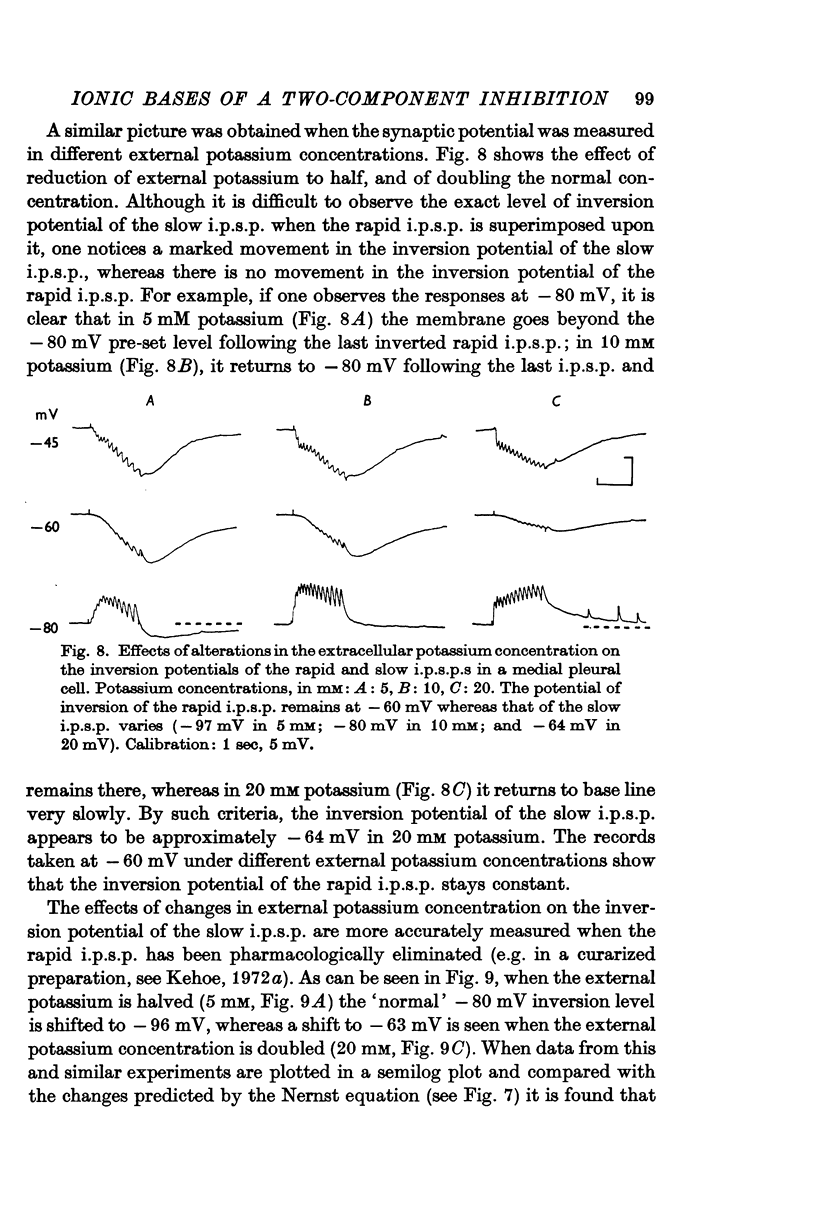
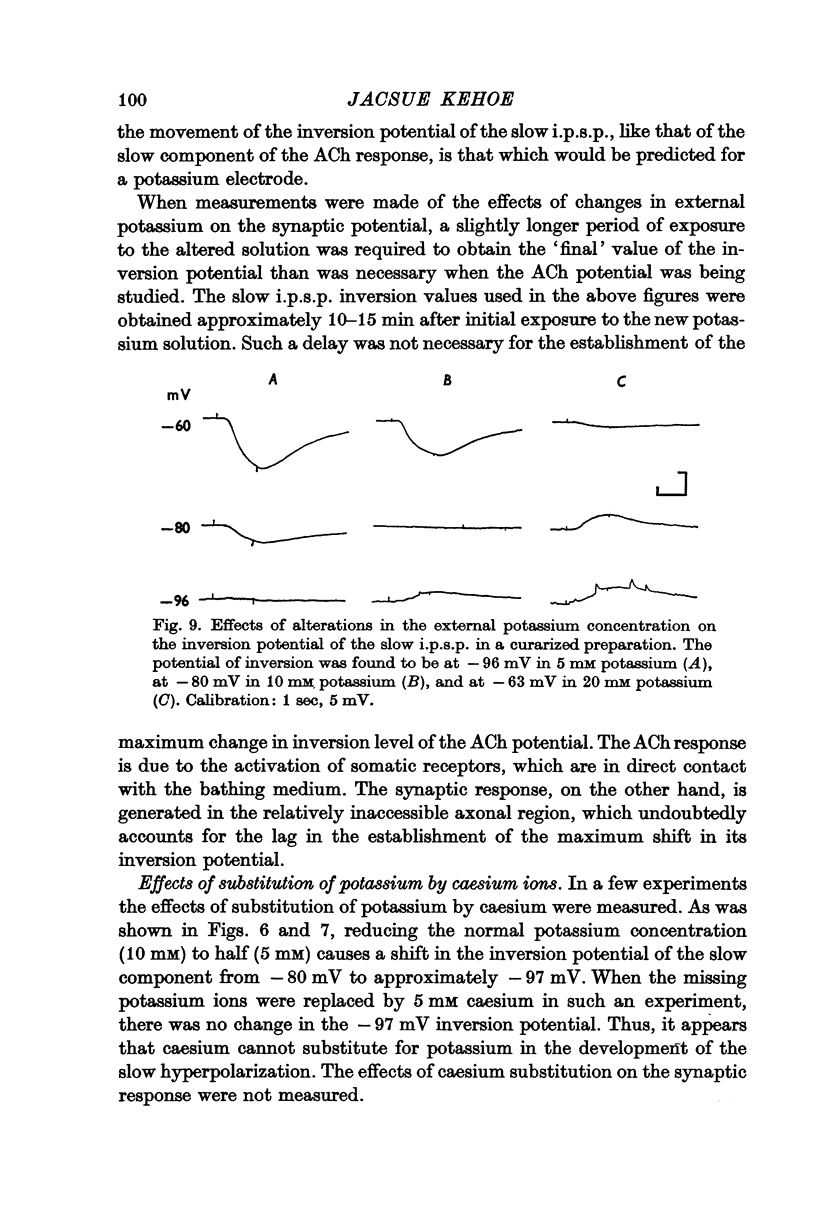
3. The slow i.p.s.p. and the slow response to ACh injection (both of which invert around -80 mV) are insensitive to changes in either internal or external chloride concentrations; on the contrary, with alterations of the concentration of potassium in the external medium, the inversion potential of the slow responses is altered in a way similar to that expected for the potassium equilibrium potential (EK).
4. It is concluded that the rapid i.p.s.p. and the corresponding ACh potential are due to a change in chloride permeability of the post-synaptic membrane, whereas the slow responses are due to a selective change in potassium permeability.
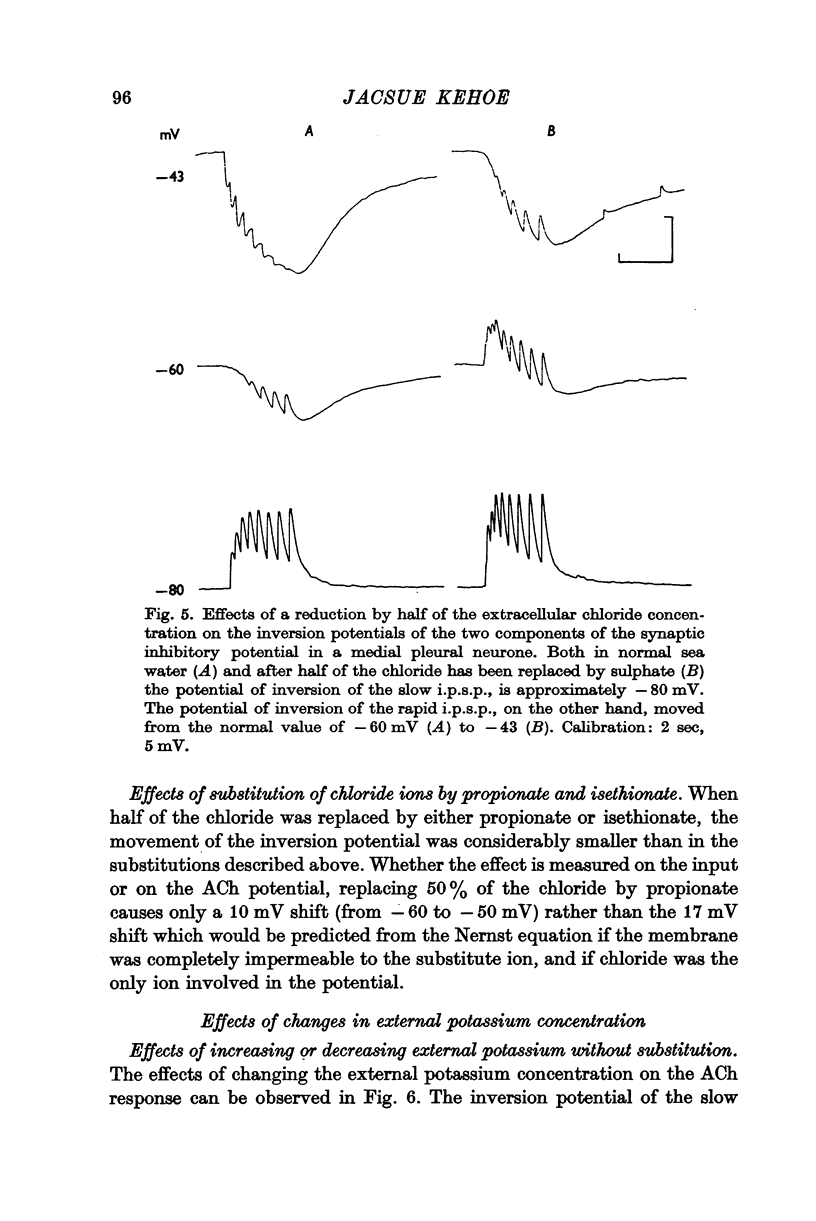
5. Additional data suggest that the fast, `chloride' channel is impermeable to sulphate and methylsulphate, but slightly permeable to propionate and isethionate. The slow, `potassium' channel is impermeable to caesium ions, whereas its permeability to rubidium ions is half that to potassium.
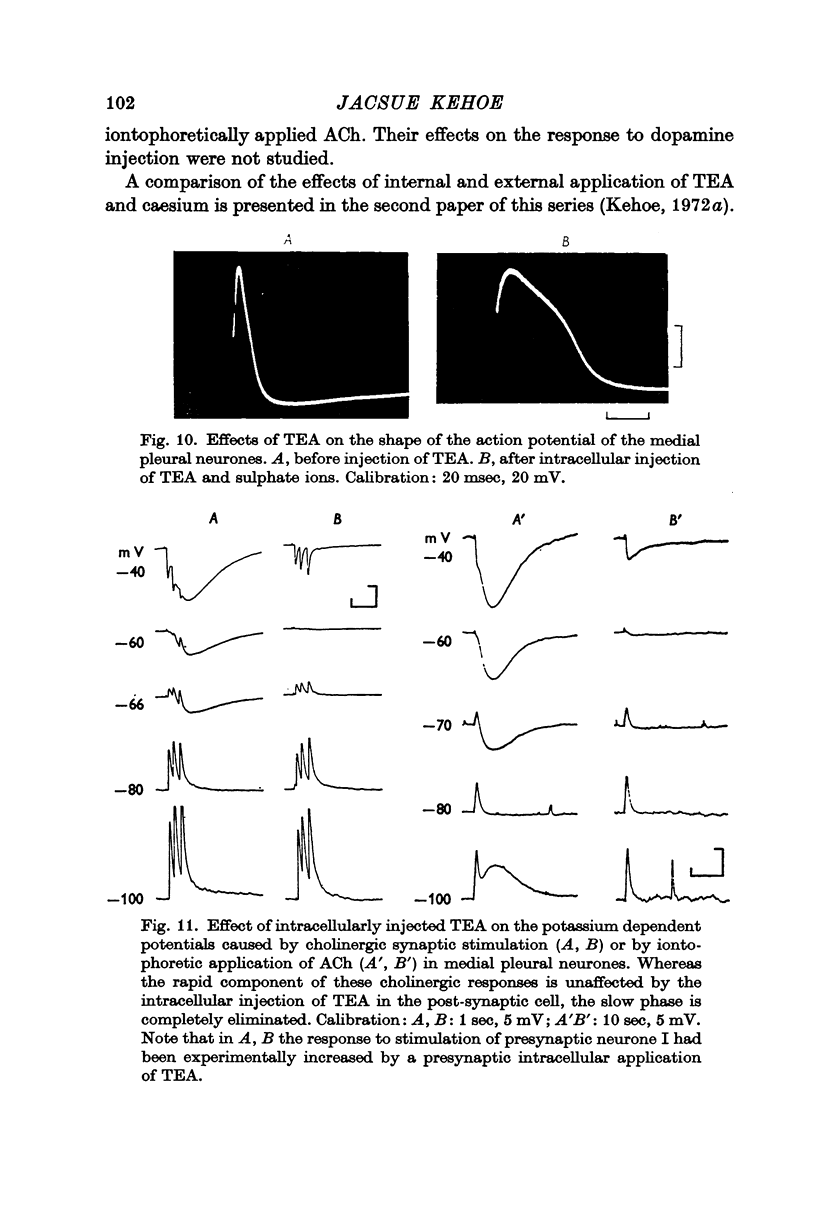
6. The potassium permeability of both the non-synaptic and synaptic membrane is markedly reduced by an intracellular injection of either tetraethylammonium (TEA) or caesium. These ions not only block the cholinergic potassium currents (whether inward or outward) but likewise block the potassium currents activated in the same cells by an iontophoretic injection of dopamine.
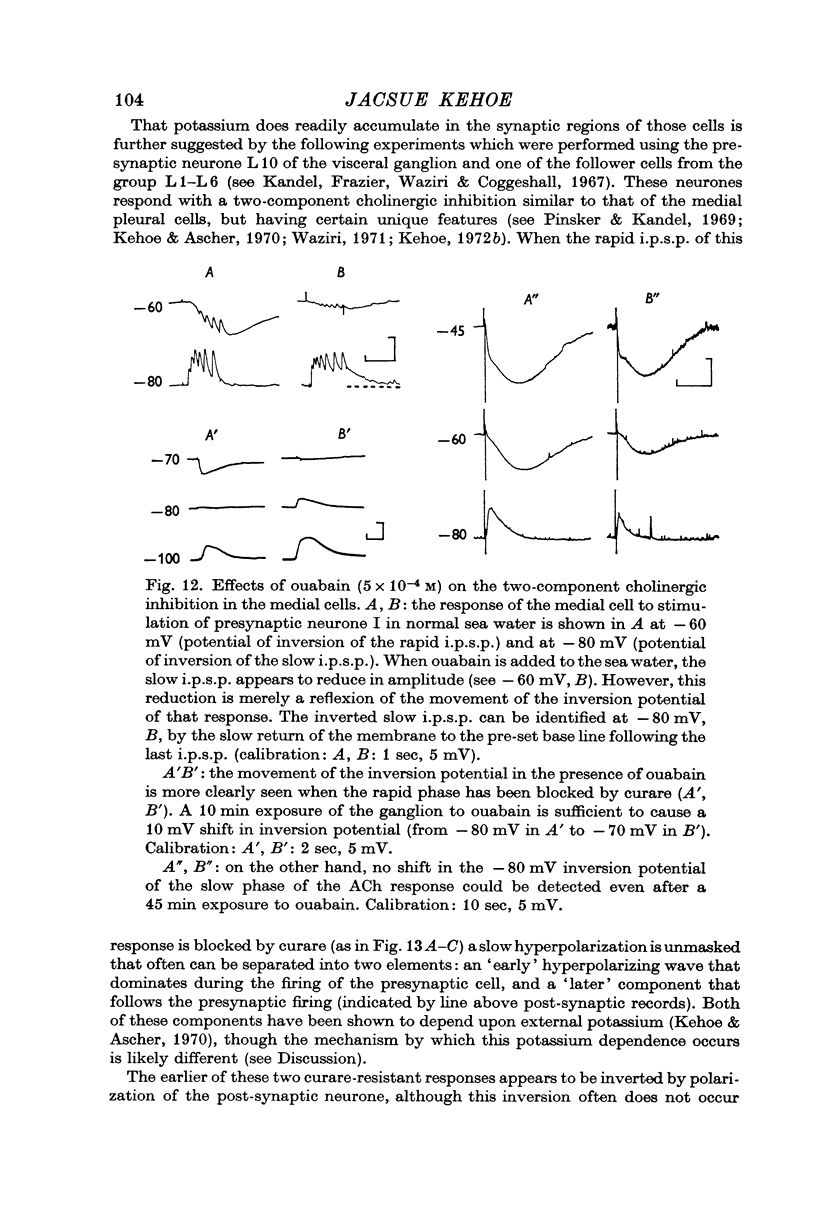
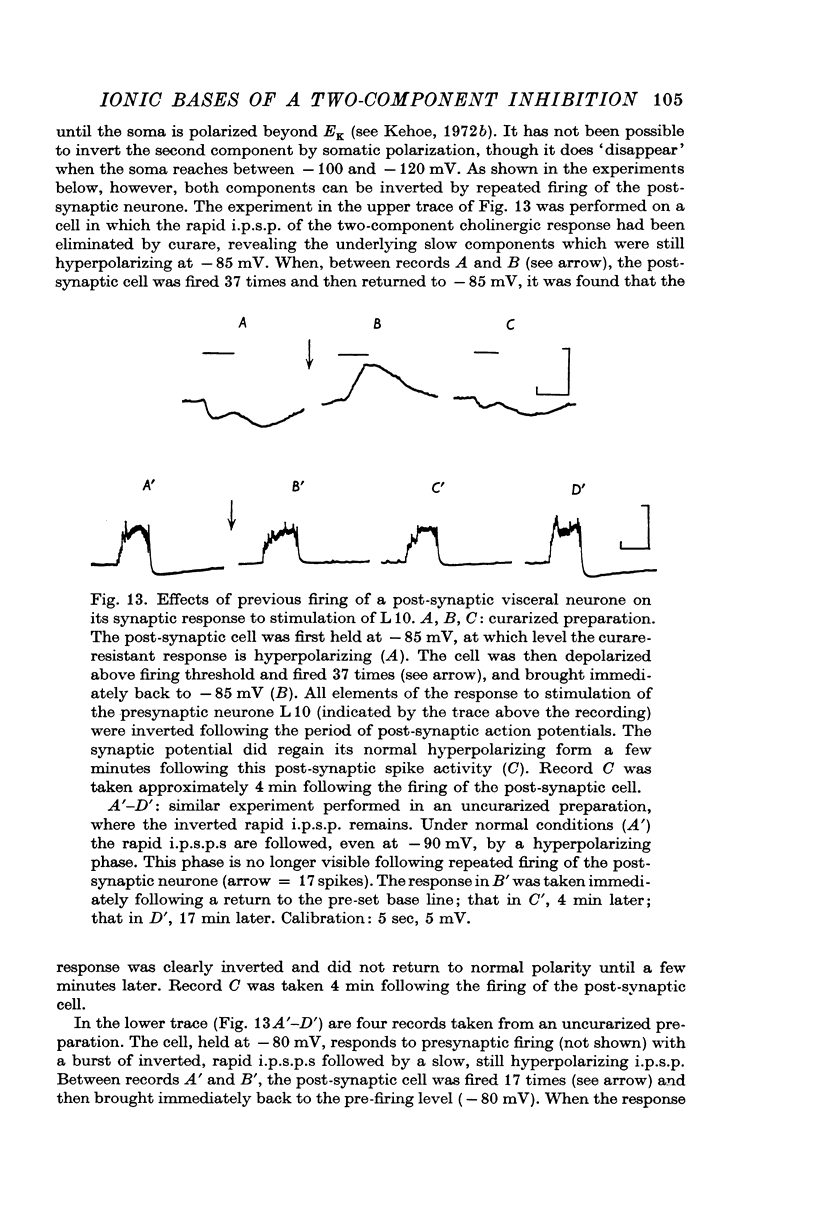
7. The potassium dependent synaptic potentials are also selectively affected by manipulations known to block the electrogenic sodium pump. In the presence of ouabain or in sea water in which sodium has been replaced by lithium, there is an apparent reduction of these potentials which was shown to be simply a reflexion of the movement of EK towards a less polarized level. This shift in inversion potential was not seen for the potassium dependent response to ACh iontophoretic injection. These results are interpreted in terms of accumulation of potassium ions assumed to occur in the extracellular spaces of the neuropile, but not in the thoroughly dissected somatic region.
8. Cooling was shown to eliminate, selectively, the synaptic and ACh potential changes caused by an increase in potassium permeability.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S., TERROUX K. G. On the negative inotropic effect in the cat's auricle. J Physiol. 1953 Jun 29;120(4):449–464. doi: 10.1113/jphysiol.1953.sp004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. E., Wachtel H., Kandel E. R. Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol. 1971 Jan;34(1):76–92. doi: 10.1152/jn.1971.34.1.76. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., HAGIWARA S. Potassium ions and the inhibitory process in the crayfish stretch receptor. J Gen Physiol. 1959 Nov;43:315–321. doi: 10.1085/jgp.43.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. The ionic mechanisms of excitatory and inhibitory synaptic action. Ann N Y Acad Sci. 1966 Jul 14;137(2):473–494. doi: 10.1111/j.1749-6632.1966.tb50176.x. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., CHIARANDINI D. J. IONIC MECHANISM ASSOCIATED WITH NON-CHOLINERGIC SYNAPTIC INHIBITION IN MOLLUSCAN NEURONS. J Neurophysiol. 1965 Jul;28:710–723. doi: 10.1152/jn.1965.28.4.710. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO S. Membrane changes in crayfish stretch receptor neutron during synaptic inhibition and under action of gamma-aminobutyric acid. J Neurophysiol. 1960 Sep;23:505–515. doi: 10.1152/jn.1960.23.5.505. [DOI] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. THE EFFECT OF ANION INJECTION AND CHANGES IN THE EXTERNAL POTASSIUM AND CHLORIDE CONCENTRATION ON THE REVERSAL POTENTIALS OF THE IPSP AND ACETYLCHOLINE. Comp Biochem Physiol. 1964 Feb;11:199–213. doi: 10.1016/0010-406x(64)90163-x. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Ascher P. Re-evaluation of the synaptic activation of an electrogenic sodium pump. Nature. 1970 Feb 28;225(5235):820–823. doi: 10.1038/225820a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. S. Suppression sélective par l'ion tétraéthylammonium d'une inhibition cholinergique résistant au curare. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 6;268(1):111–114. [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Brown A. M. Internal potassium and chloride activities and the effects of acetylcholine on identifiable Aplysia neurones. Nat New Biol. 1971 Feb 24;229(8):229–231. doi: 10.1038/newbio229229a0. [DOI] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Pinsker H., Kandel E. R. Synaptic activation of an electrogenic sodium pump. Science. 1969 Feb 28;163(3870):931–935. doi: 10.1126/science.163.3870.931. [DOI] [PubMed] [Google Scholar]
- Sato M., Austin G., Yai H., Maruhashi J. The ionic permeability changes during acetylcholine-induced responses of Aplysia ganglion cells. J Gen Physiol. 1968 Mar;51(3):321–345. doi: 10.1085/jgp.51.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M. [Ionic mechanisms of the activated subsynaptic membrane in Onchidium neurons]. Nihon Seirigaku Zasshi. 1969 Aug 31;31(8):491–504. [PubMed] [Google Scholar]
- TRAUTWEIN W., DUDEL J. Zum Mechanismus der Membranwirkung des Acetylcholin an der Herzmuskelfaser. Pflugers Arch. 1958;266(3):324–334. doi: 10.1007/BF00416781. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]


