Abstract
1. The cerebellar integration of sensory inputs to Deiters neurones was studied in cats under Nembutal anaesthesia.
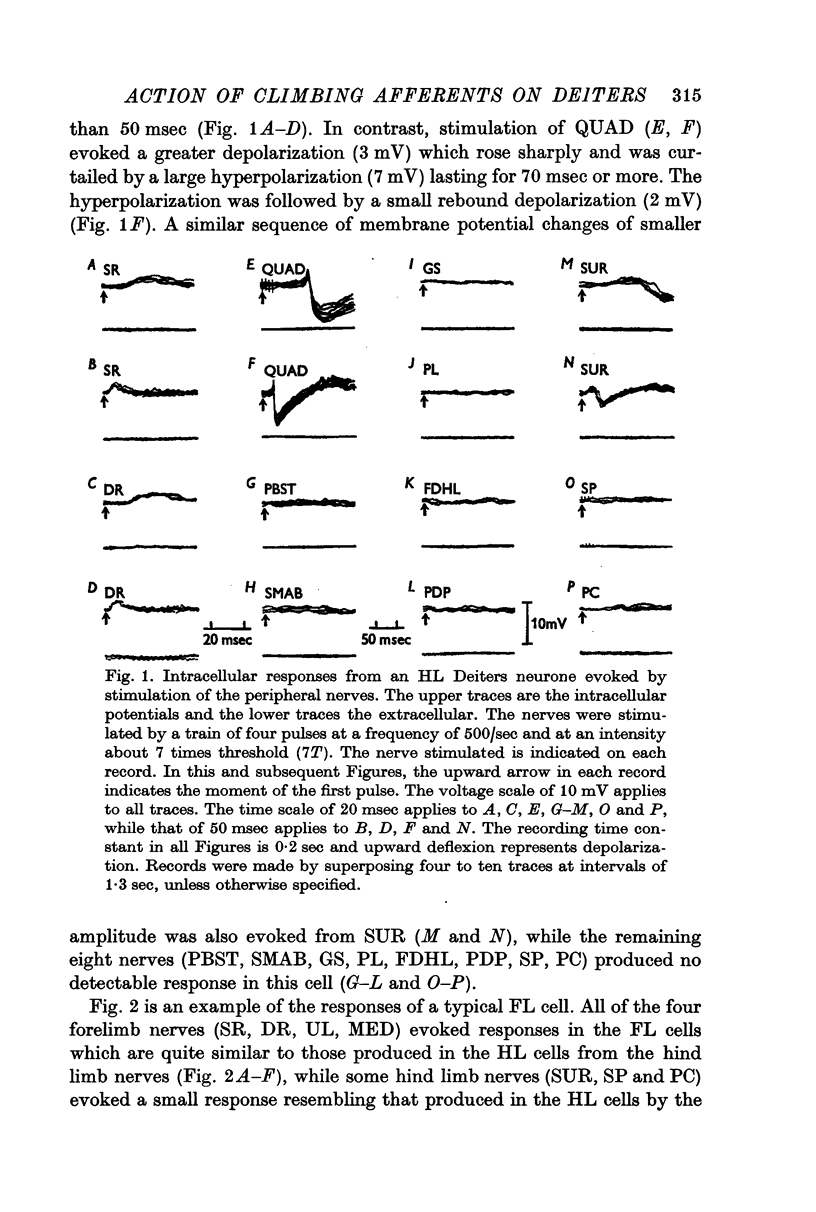
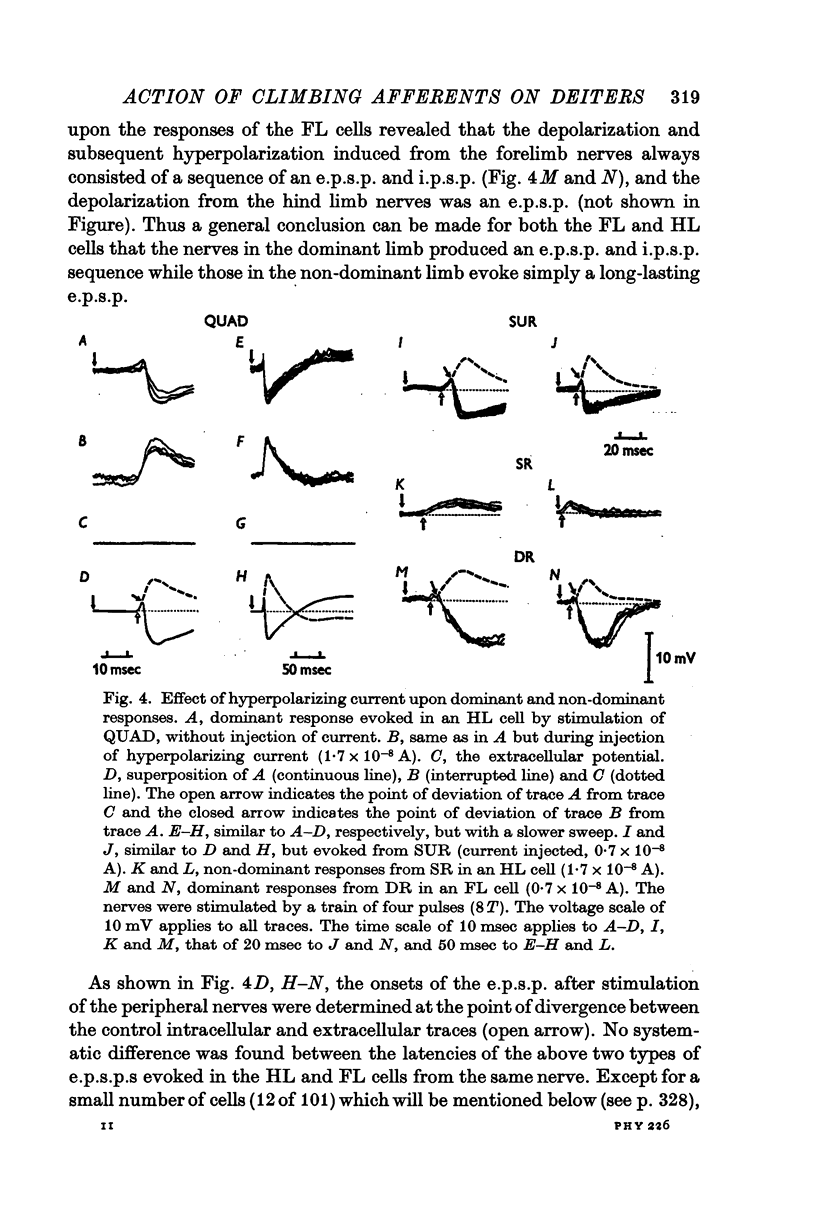
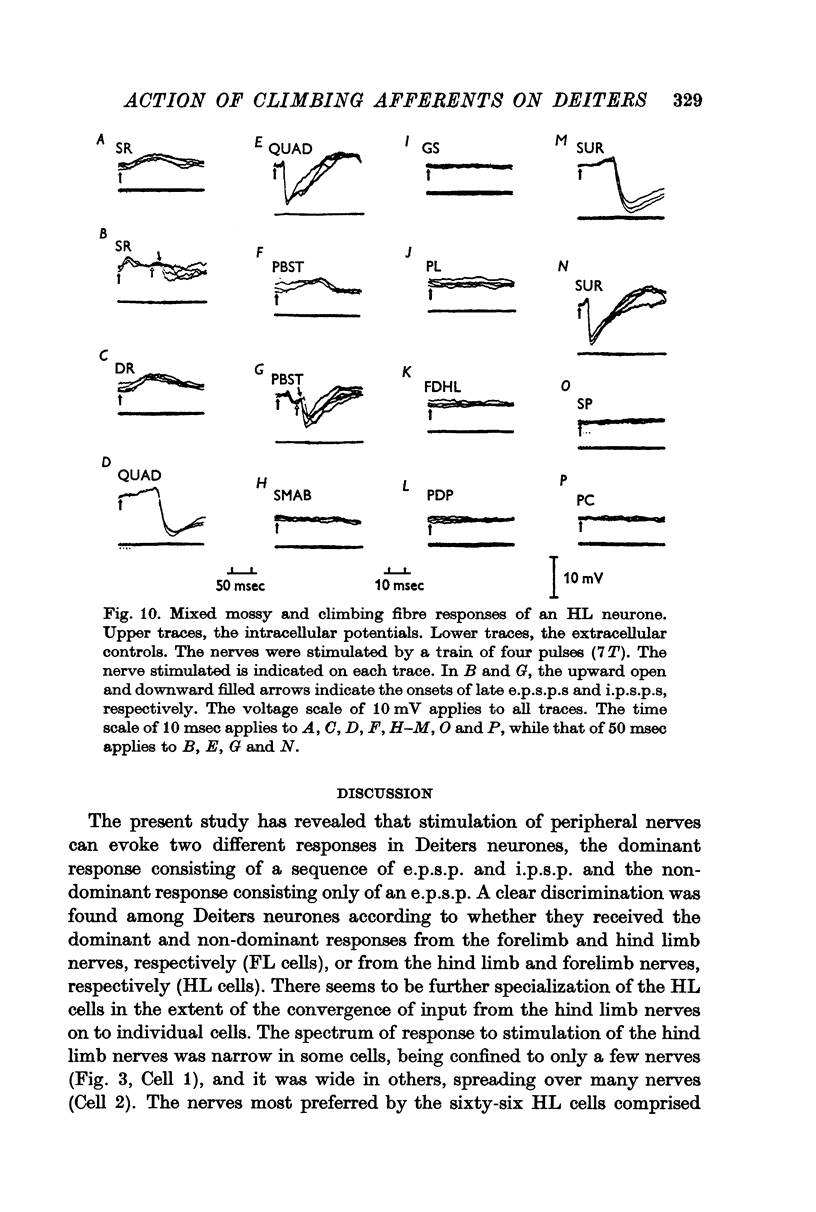
2. Stimulation of peripheral nerves produced in the Deiters neurones a sequence of an initial excitatory post-synaptic potential (e.p.s.p.) and a later inhibitory post-synaptic potential (i.p.s.p.), or a relatively small e.p.s.p.
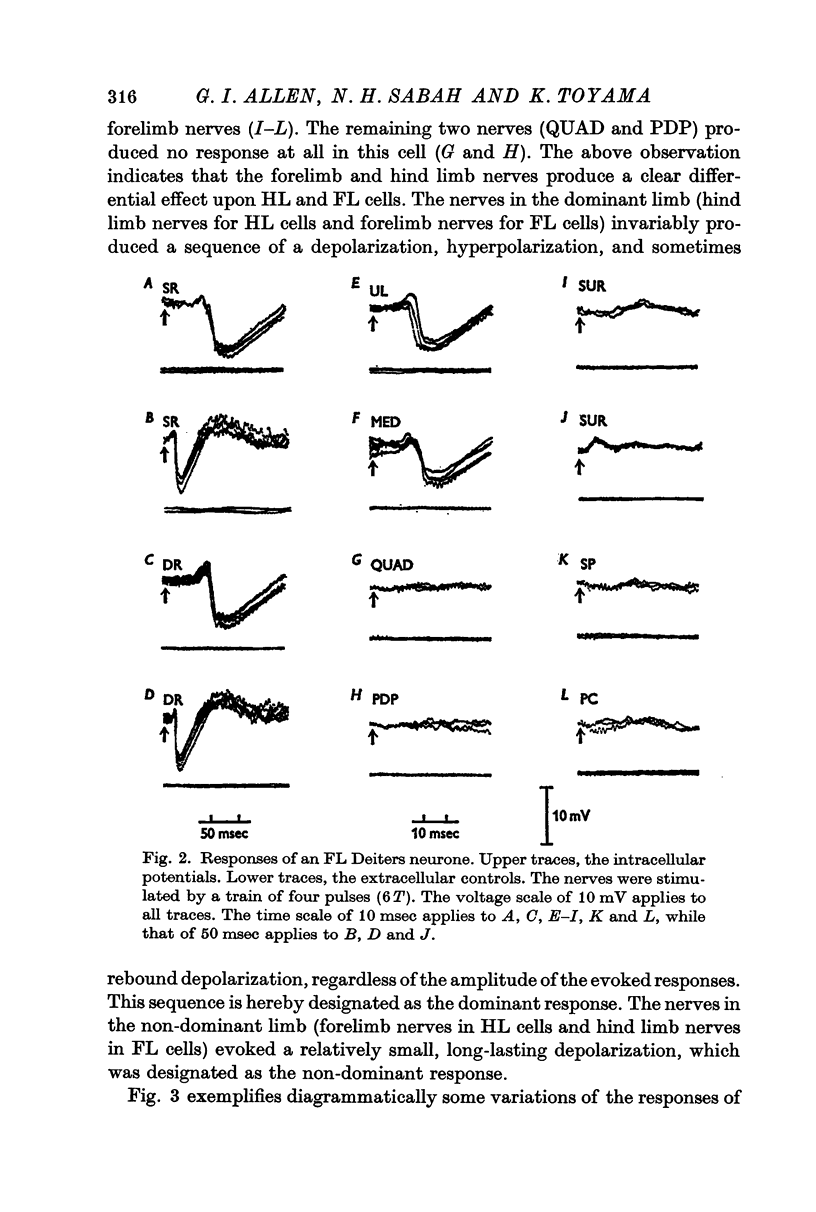
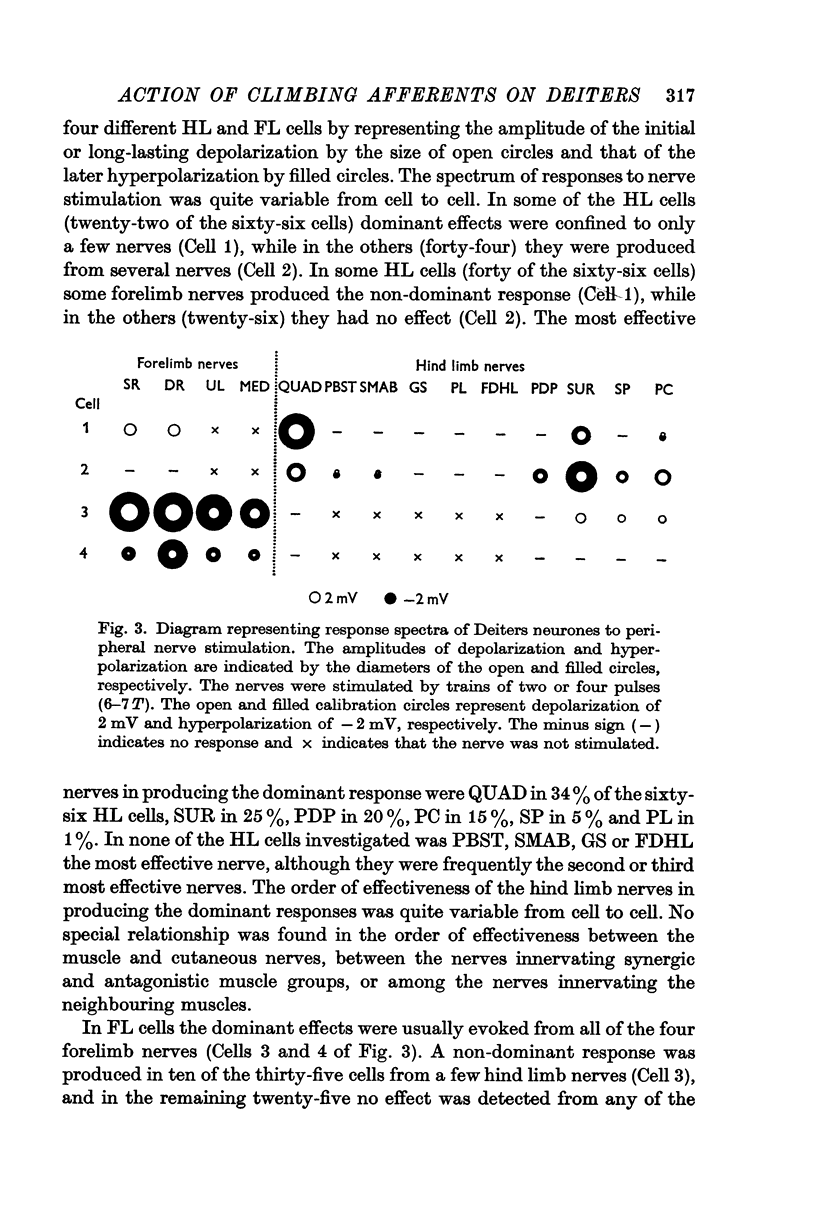
3. The Deiters neurones were classified as forelimb (FL)- or hind limb (HL)-type cells according to the location of the most effective peripheral nerve. In the FL cells stimulation of the forelimb nerves produced the e.p.s.p.—i.p.s.p. sequence (dominant response), while stimulation of the hind limb nerves was ineffective or produced the small e.p.s.p. (non-dominant response). In contrast, in the HL cells the non-dominant response was evoked from the forelimb nerves, and the dominant response from the hind limb nerves.
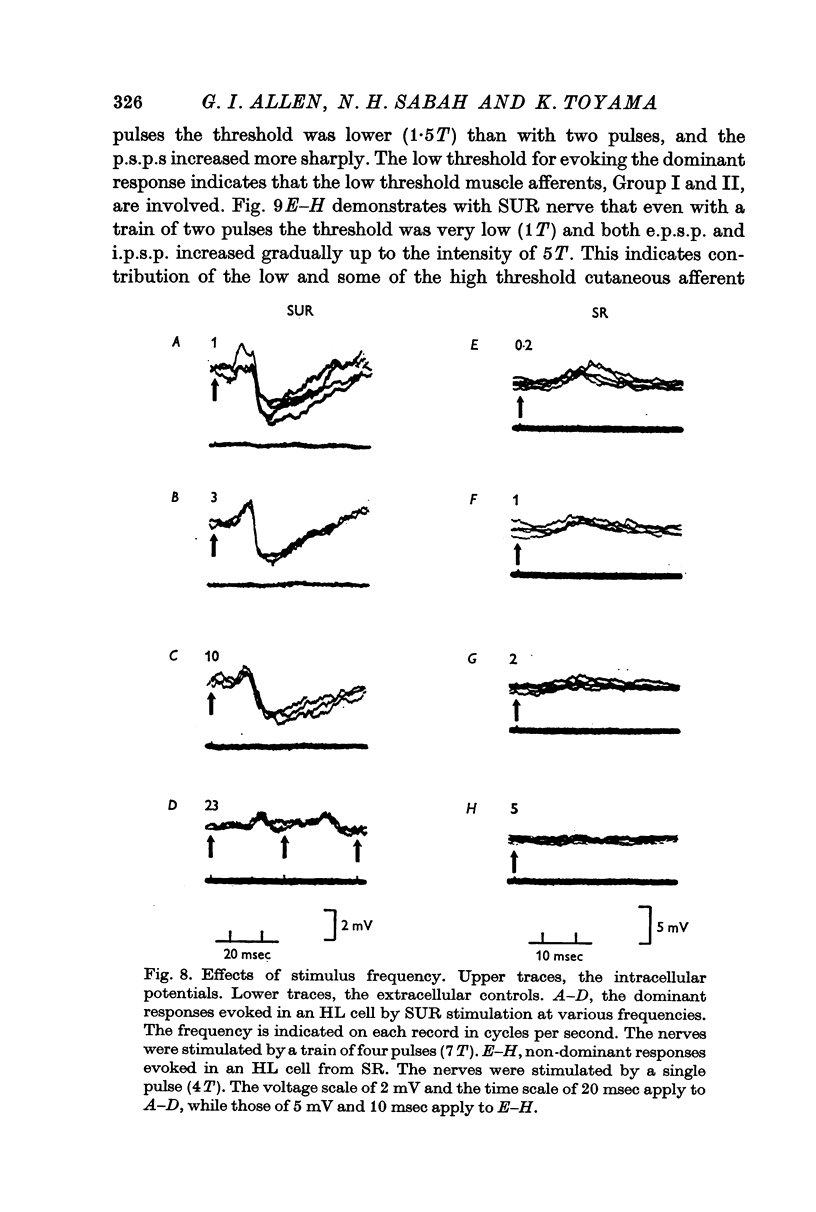
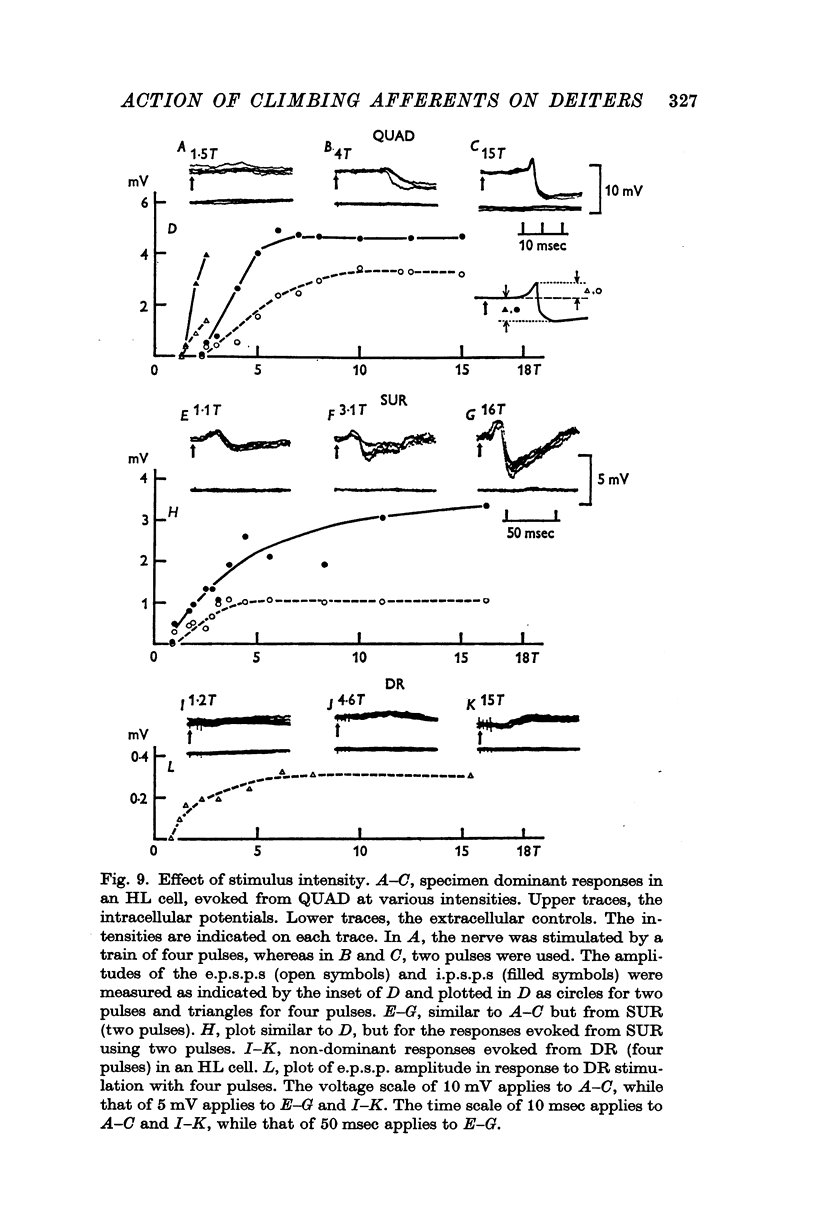
4. The stimulus intensity—response relation indicates that Group I and II muscle afferents and low and high threshold cutaneous afferents contribute to the dominant and non-dominant responses.
5. Antidromic identification of these Deiters neurones revealed that 90% of the HL cells and 85% of the FL cells project to the lumbo-sacral and cervico-thoracic segments of the spinal cord, respectively, while 10% of the HL cells and 15% of the FL cells innervate the cervico-thoracic and lumbo-sacral segments, respectively.
6. The mean latency of the e.p.s.p. evoked from the forelimb nerves was 14 msec in the FL cells and 13 msec in the HL cells, and the latency of the e.p.s.p. evoked from the hind limb nerves was 17 msec in the FL cells and 18 msec in the HL cells. The later i.p.s.p. regularly followed the onset of the e.p.s.p. with a delay of 3-5 msec.
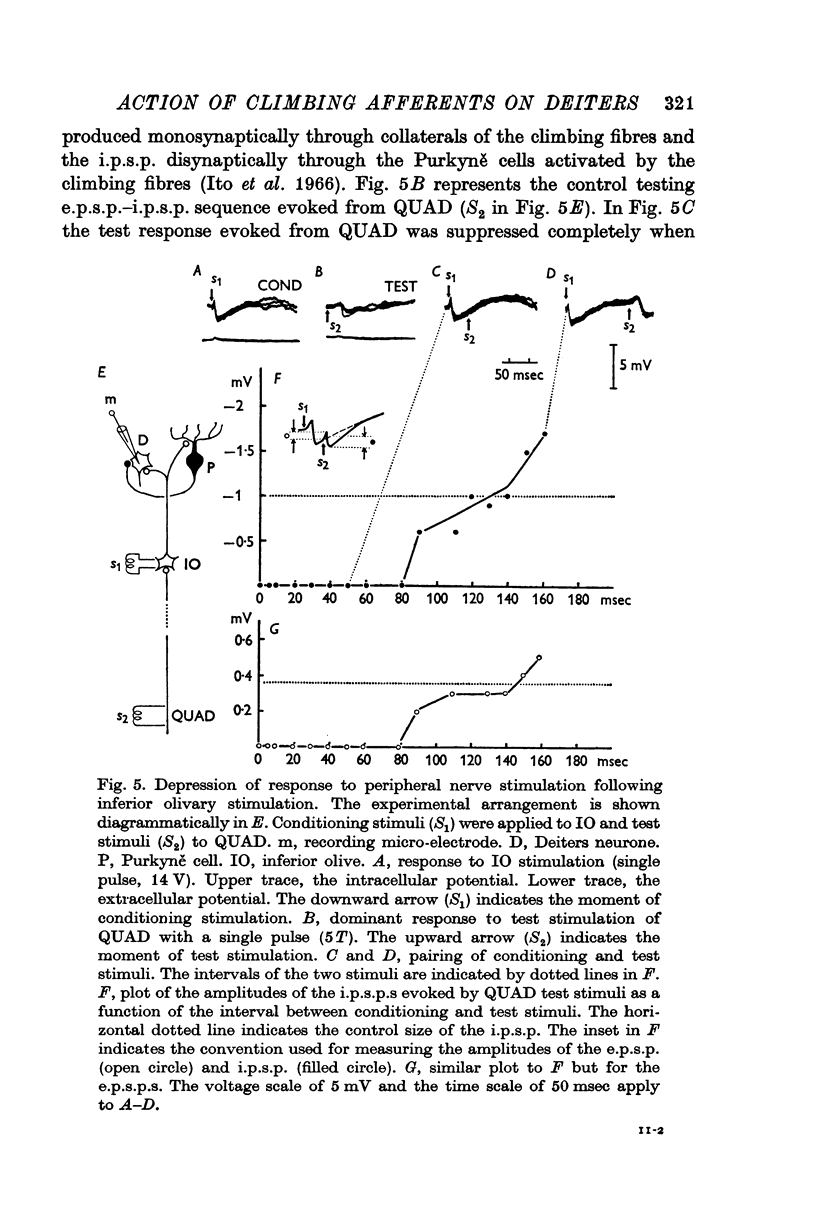
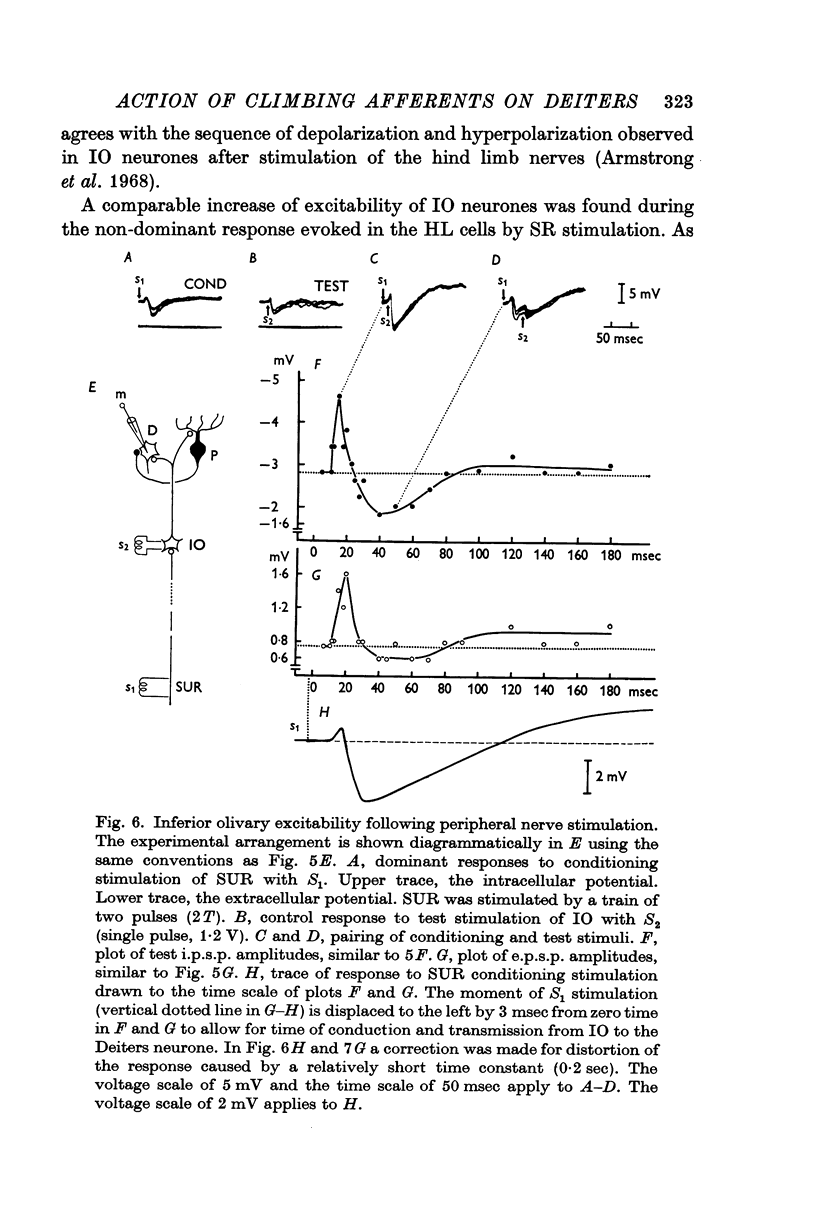
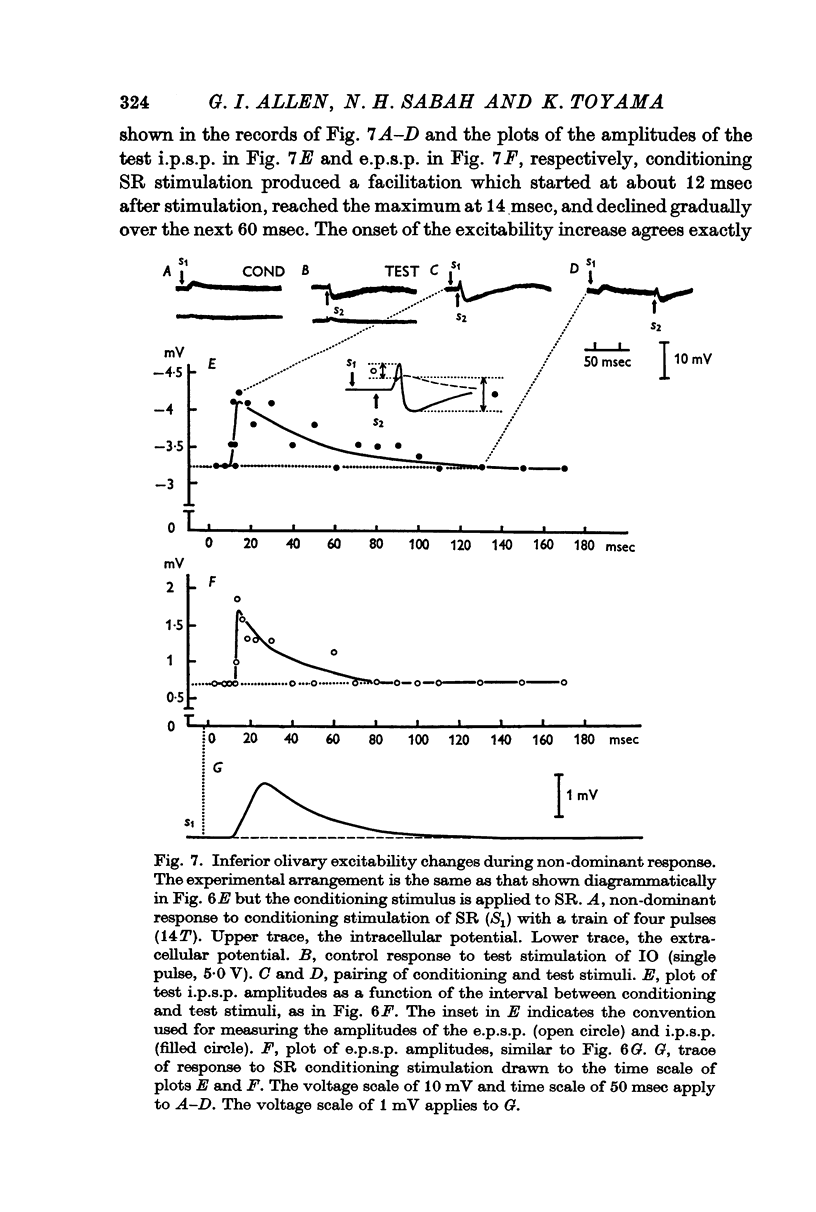
7. The dominant and non-dominant responses in both types of cells exhibited the following three characteristic features: (i) a strong depression after conditioning stimulation of the inferior olive, (ii) an increase of the inferior olivary excitability during the responses, and (iii) a striking frequency depression with stimulation at relatively low frequency (5-10/sec).
8. Consequently it was concluded that all of the responses were produced through the climbing fibres originating from the inferior olive, the i.p.s.p.s due to inhibition from Purkyně cells activated by the climbing fibres and the e.p.s.p.s due to excitation from the collaterals of the climbing fibres.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Sabah N. H., Toyama K. Effect of fore- and hindlimb nerve stimulation on Deiters' neurones. Brain Res. 1971 Feb 5;25(3):645–650. doi: 10.1016/0006-8993(71)90469-0. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Sabah N. H., Toyama K. Synaptic actions of peripheral nerve impulses upon Deiters neurones via the mossy fibre afferents. J Physiol. 1972 Oct;226(2):335–351. doi: 10.1113/jphysiol.1972.sp009987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E. Unitary multiple-spiked responses in cat inferior olive nucleus. J Neurophysiol. 1970 Mar;33(2):199–209. doi: 10.1152/jn.1970.33.2.199. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkyne cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Firing patterns of Purkinje cells in response to volleys from limb nerves. Brain Res. 1969 Jun;14(1):222–226. doi: 10.1016/0006-8993(69)90043-2. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Investigations on integration of mossy fiber inputs to Purkynè cells in the anterior lobe. Exp Brain Res. 1971 Jul 26;13(1):54–77. [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Topographical investigations on the climbing fiber inputs from forelimb and hindlimb afferents to the cerebellar anterior lobe. Exp Brain Res. 1968;6(3):195–215. doi: 10.1007/BF00235124. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. The vestibulospinal tract. Effects on alpha-motoneurones in the lumbosacral spinal cord in the cat. Exp Brain Res. 1970;10(1):94–120. doi: 10.1007/BF00340521. [DOI] [PubMed] [Google Scholar]
- ITO M., HONGO T., YOSHIDA M., OKADA Y., OBATA K. ANTIDROMIC AND TRANS-SYNAPTIC ACTIVATION OF DEITERS' NEURONES INDUCED FROM THE SPINAL CORD. Jpn J Physiol. 1964 Dec 15;14:638–658. doi: 10.2170/jjphysiol.14.638. [DOI] [PubMed] [Google Scholar]
- Ito M., Hongo T., Okada Y. Vestibular-evoked postsynaptic potentials in Deiters neurones. Exp Brain Res. 1969;7(3):214–230. doi: 10.1007/BF00239030. [DOI] [PubMed] [Google Scholar]
- Ito M., Kawai N., Udo M., Mano N. Axon reflex activation of Deiters neurones from the cerebellar cortex through collaterals of the cerebellar afferents. Exp Brain Res. 1969;8(3):249–268. doi: 10.1007/BF00234252. [DOI] [PubMed] [Google Scholar]
- Ito M., Kawai N., Udo M., Sato N. Cerebellar-evoked disinhibition in dorsal Deiters neurones. Exp Brain Res. 1968;6(3):247–264. doi: 10.1007/BF00235127. [DOI] [PubMed] [Google Scholar]
- Ito M., Obata K., Ochi R. The origin of cerebellar-induced inhibition of Deiters neurones. II. Temporal correlation between the trans-synaptic activation of Purkinje cells and the inhibition of Dieters neurones. Exp Brain Res. 1966;2(4):350–364. doi: 10.1007/BF00234780. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M. The origin of cerebral-induced inhibition of Deiters neurones. I. Monosynaptic initiation of the inhibitory postsynaptic potentials. Exp Brain Res. 1966;2(4):330–349. doi: 10.1007/BF00234779. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]
- Wilson V. J., Yoshida M., Schor R. H. Supraspinal monosynaptic excitation and inhibition of thoracic back montoneurons. Exp Brain Res. 1970;11(3):282–295. doi: 10.1007/BF01474387. [DOI] [PubMed] [Google Scholar]


