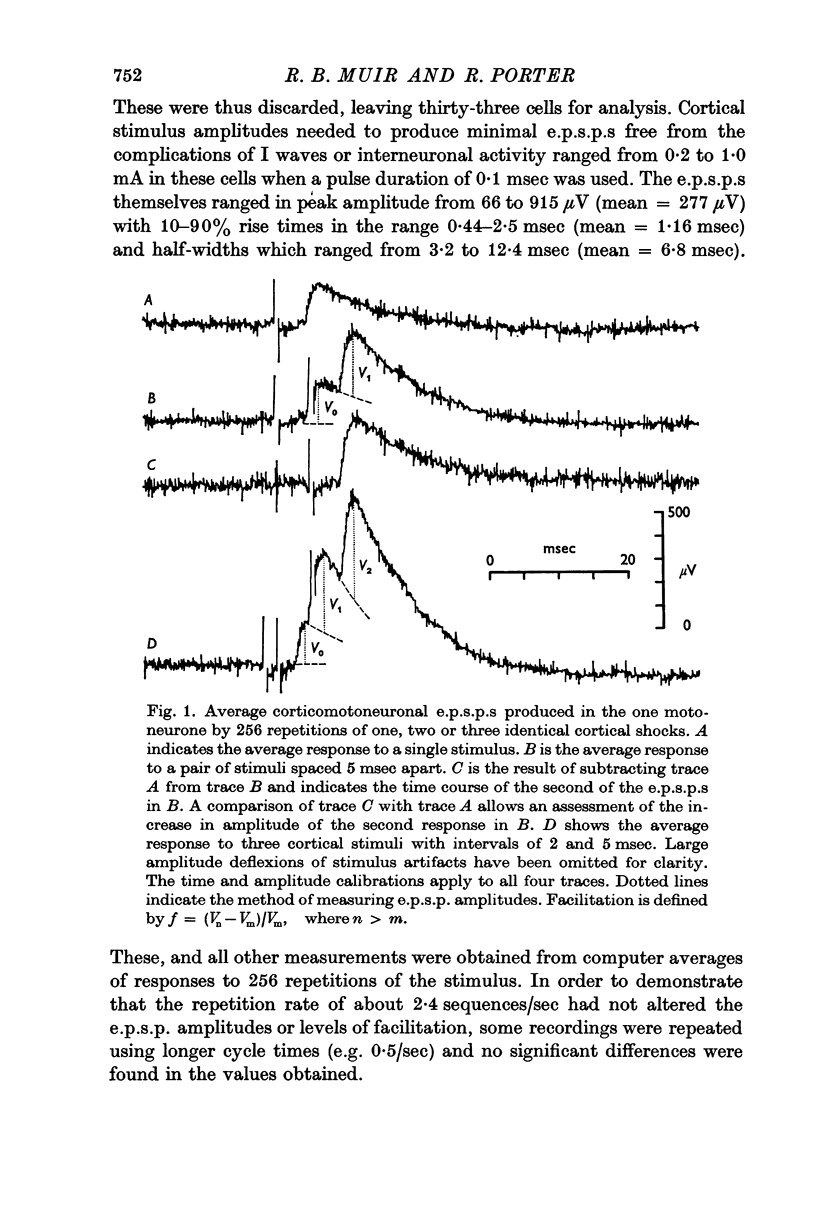
Abstract
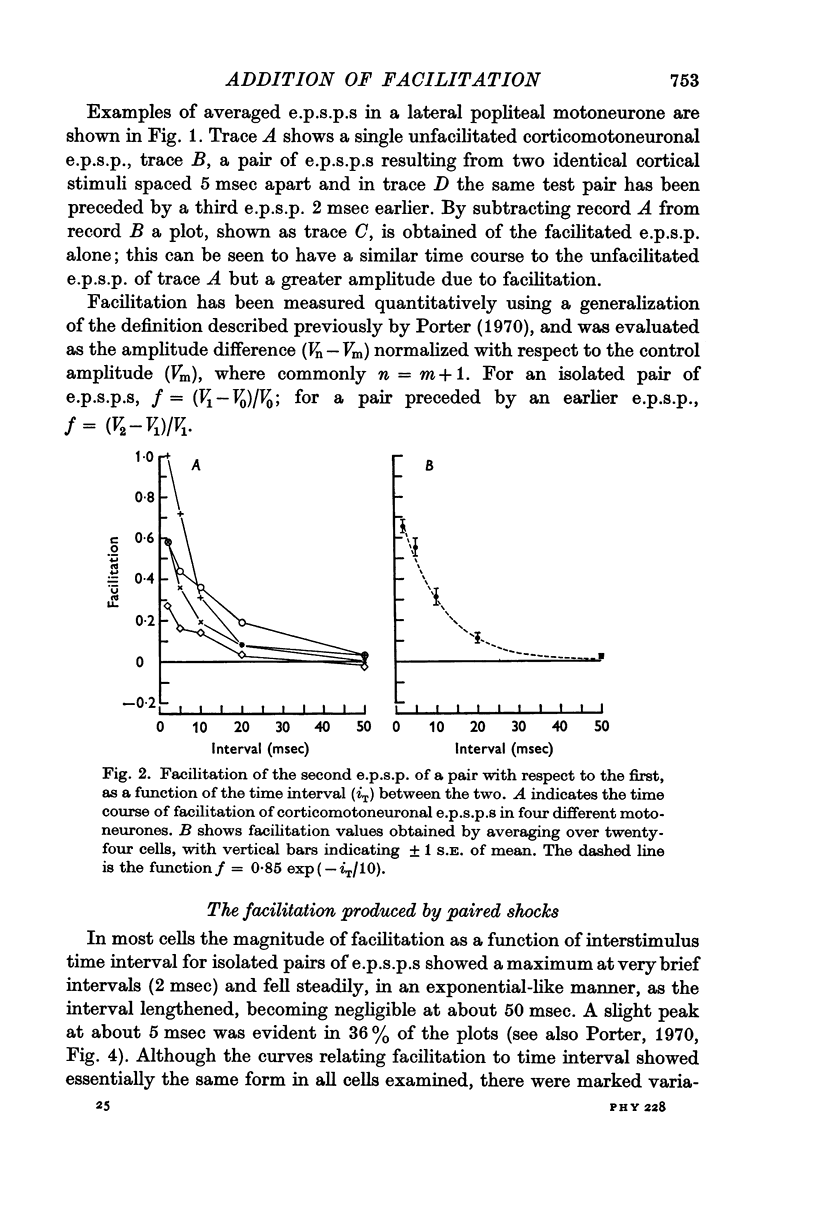
1. Intracellular recordings were made of minimal corticomotoneuronal e.p.s.p.s in lumbar motoneurones of anaesthetized monkeys. For intervals of 2 msec and greater between paired cortical shocks, the average time course of facilitation of the second e.p.s.p. with respect to the first could be fitted closely by a negative exponential with a time constant of 10 msec.
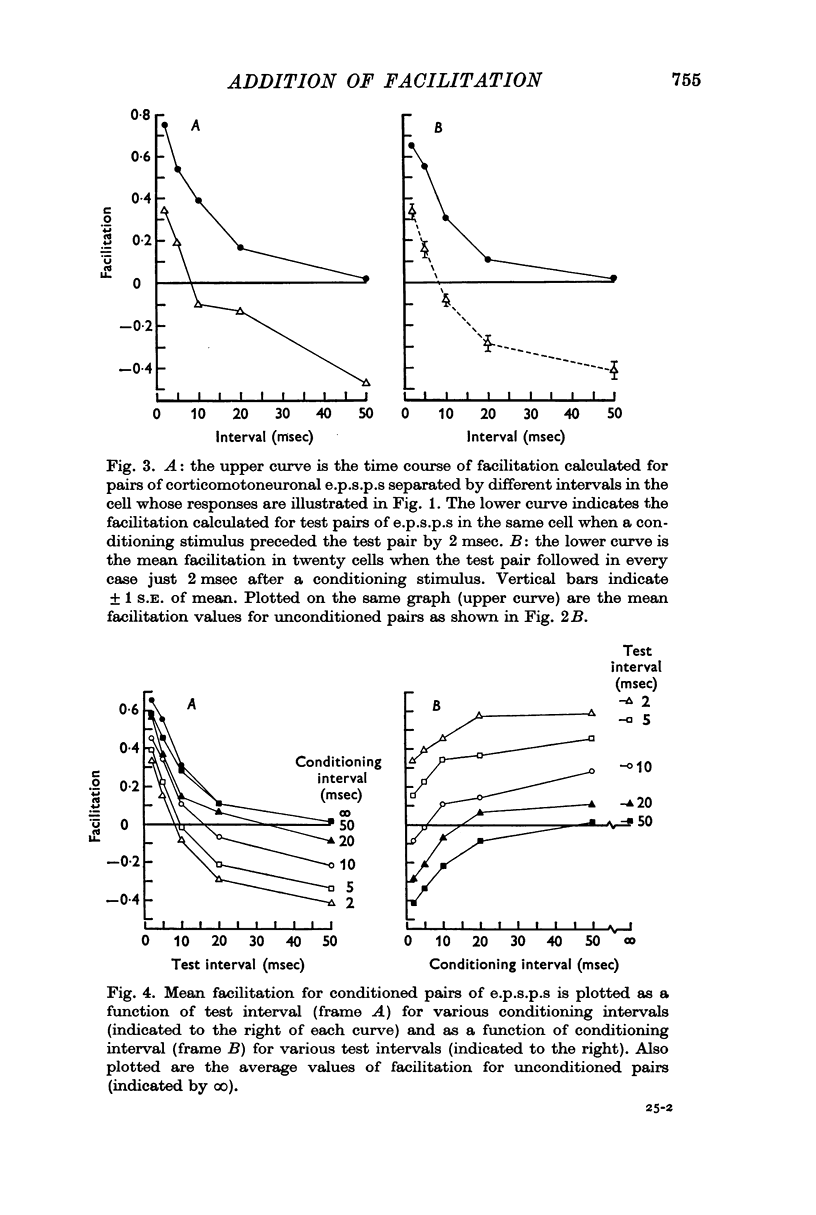
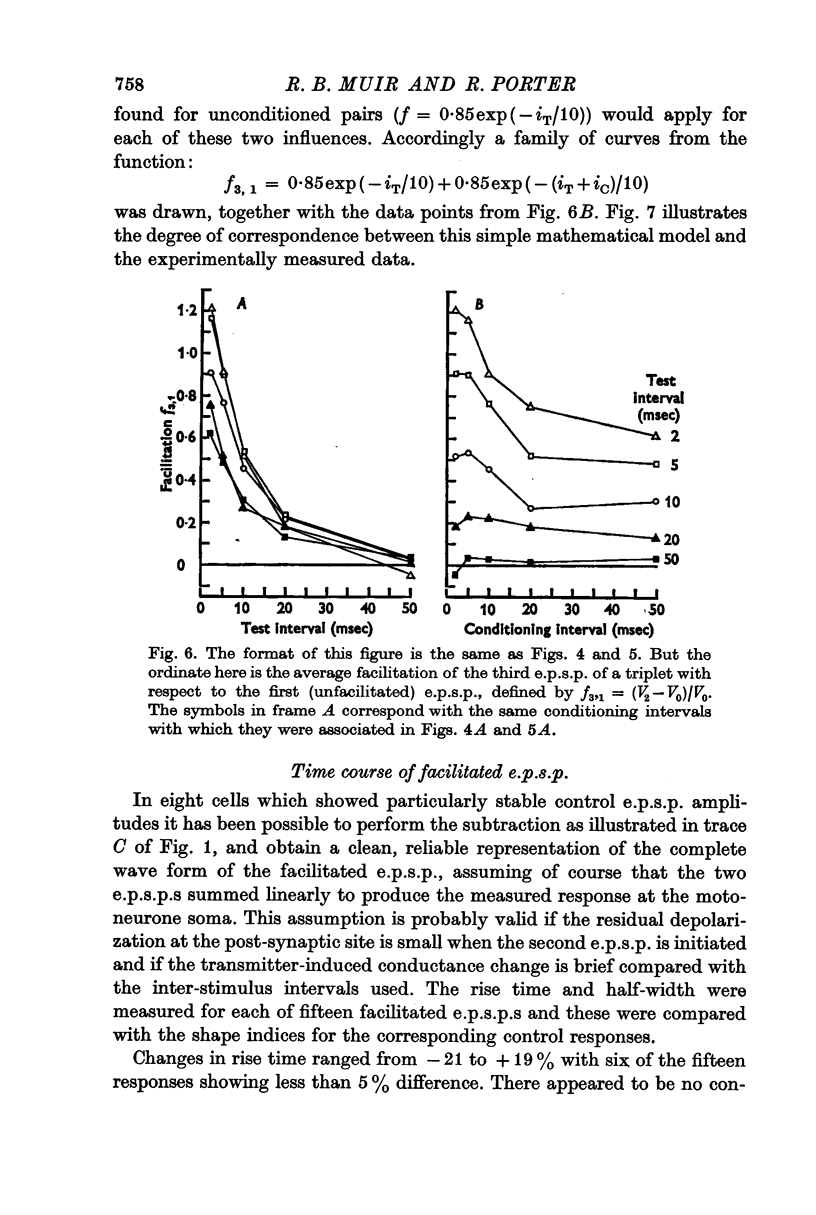
2. In the same motoneurones, `triplets' of corticomotoneuronal e.p.s.p.s were generated by delivering three identical stimuli to the motor cortex. Considering the triplet as a conditioning e.p.s.p. followed by a test pair, the facilitation of the third e.p.s.p. with respect to the second was measured for various combinations of test and conditioning intervals. In each case the amplitude of the third e.p.s.p. was also compared with that of the first (conditioning) e.p.s.p.
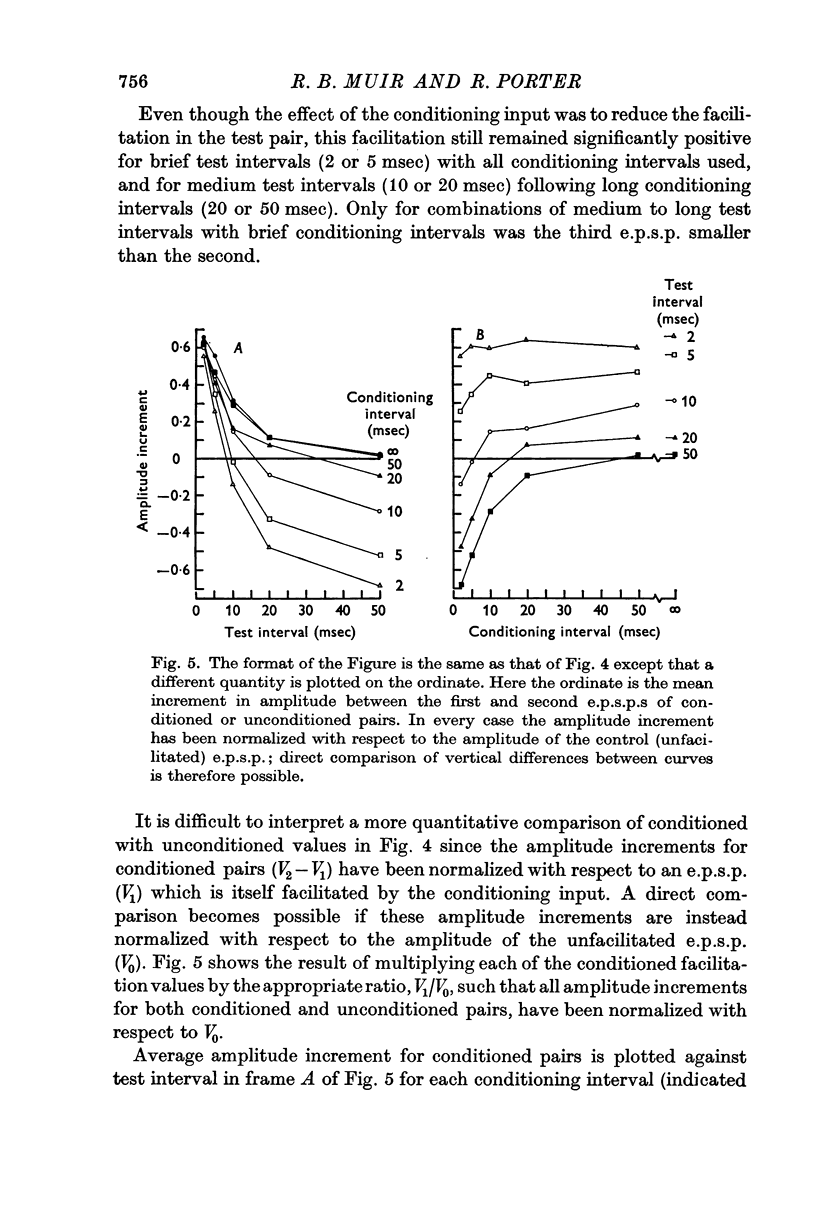
3. The effect of a brief conditioning interval was to reduce considerably the facilitation of the third e.p.s.p. with respect to the second at all test intervals from 2 to 50 msec. Combinations of brief conditioning intervals (e.g. 2 or 5 msec) and long test intervals (e.g. 20 or 50 msec) caused the third e.p.s.p. to be smaller than the second. As the conditioning interval lengthened, facilitation in the test pair increased towards the unconditioned values at all test intervals.
4. Facilitation of the third e.p.s.p. with respect to the first could be described approximately as the linear addition of two facilitation components, one due to the conditioning input and one due to the first stimulus of the test pair. Each component followed the same negative exponential time course as found for an isolated pair of e.p.s.p.s and each of the first two inputs contributed to the facilitation of the third e.p.s.p. as if the other of these two inputs had not occurred.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. Membrane time constants of cat motoneurons and time courses of synptic action. Exp Neurol. 1961 Jul;4:1–22. doi: 10.1016/0014-4886(61)90074-7. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968 Jan;31(1):14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R. The different time courses of minimal EPSPs in spinal motoneurones. J Physiol. 1967 Jul;191(2):112P–113P. [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Cortical fields of origin of the monosynaptic pyramidal pathways to some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:112–125. doi: 10.1113/jphysiol.1962.sp006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTON H. D., AMASSIAN V. E. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954 Jul;17(4):345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., PORTER R. THE PYRAMIDAL PROJECTION TO MOTONEURONES OF SOME MUSCLE GROUPS OF THE BABOON'S FORELIMB. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- Porter R. Early facilitation at corticomotoneuronal synapses. J Physiol. 1970 May;207(3):733–745. doi: 10.1113/jphysiol.1970.sp009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R., Hore J. Time course of minimal corticomotoneuronal excitatory postsynaptic potentials in lumbar motoneurons of the monkey. J Neurophysiol. 1969 May;32(3):443–451. doi: 10.1152/jn.1969.32.3.443. [DOI] [PubMed] [Google Scholar]
- Porter R., Lewis M. M., Horne M. Analysis of patterns of natural activity of neurones in the precentral gyrus of conscious monkeys. Brain Res. 1971 Nov;34(1):99–113. doi: 10.1016/0006-8993(71)90353-2. [DOI] [PubMed] [Google Scholar]
- Porter R., Muir R. B. The meaning for motoneurones of the temporal pattern of natural activity in pyramidal tract neurones of conscious monkeys. Brain Res. 1971 Nov;34(1):127–142. doi: 10.1016/0006-8993(71)90355-6. [DOI] [PubMed] [Google Scholar]
- Porter R. Relationship of the discharges of cortical neurones to movement in free-to-move monkeys. Brain Res. 1972 May 12;40(1):39–43. doi: 10.1016/0006-8993(72)90103-5. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]


