Abstract
1. The effect of background illumination on response pattern is correlated with its effect on visual sensitivity by analysing post-stimulus time-histograms obtained from single ganglion cells in the cat's retina at various levels of background illumination between zero and 2 × 106 photons (wave-length 523 nm).sec-1.deg-2 (via 5·7 mm2 pupil).
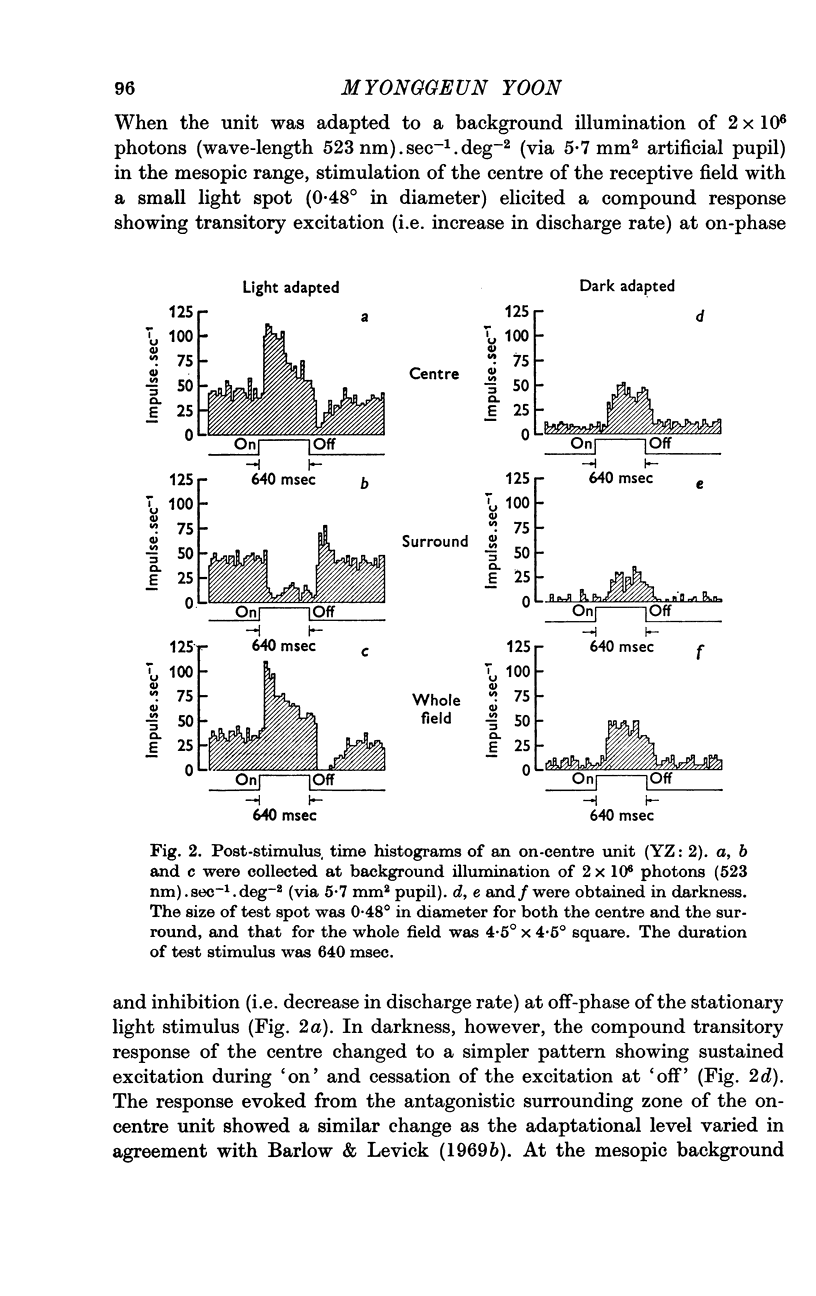
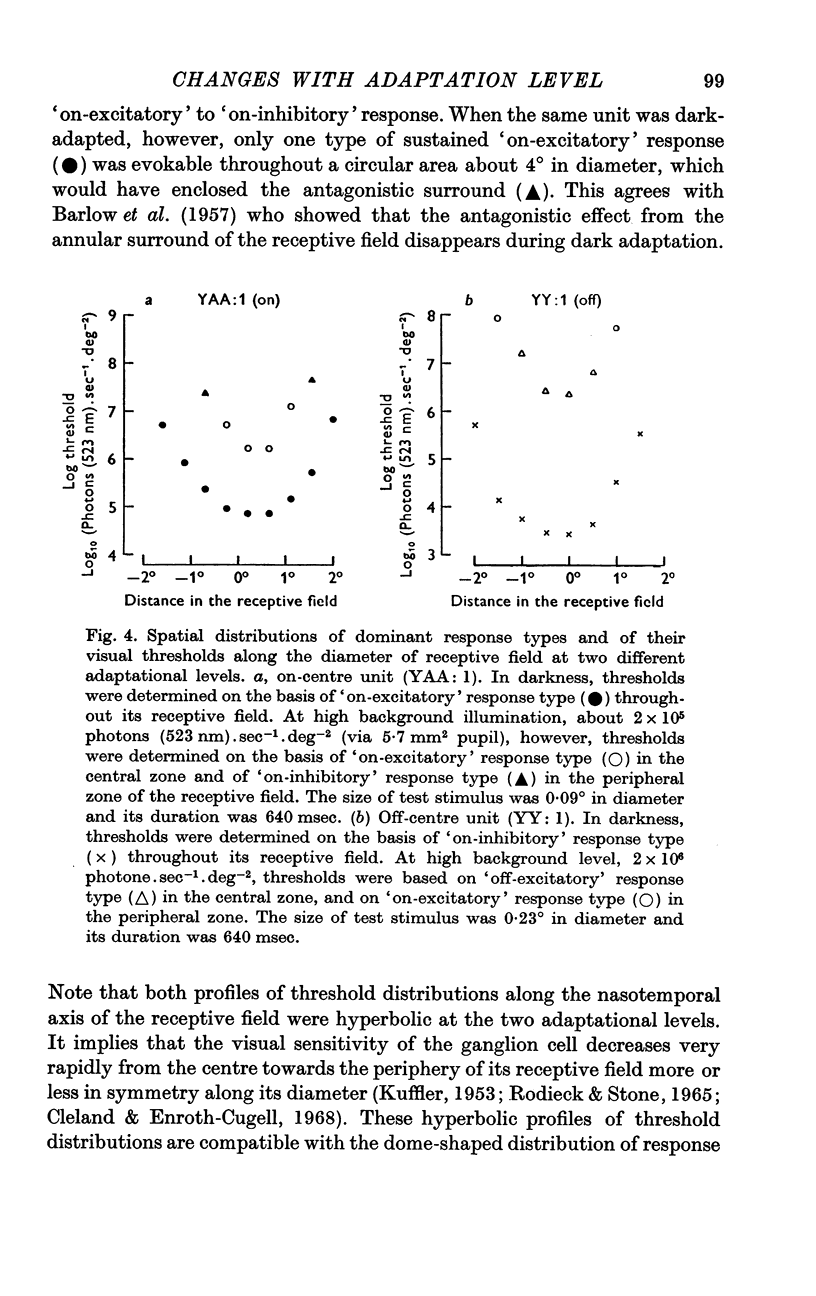
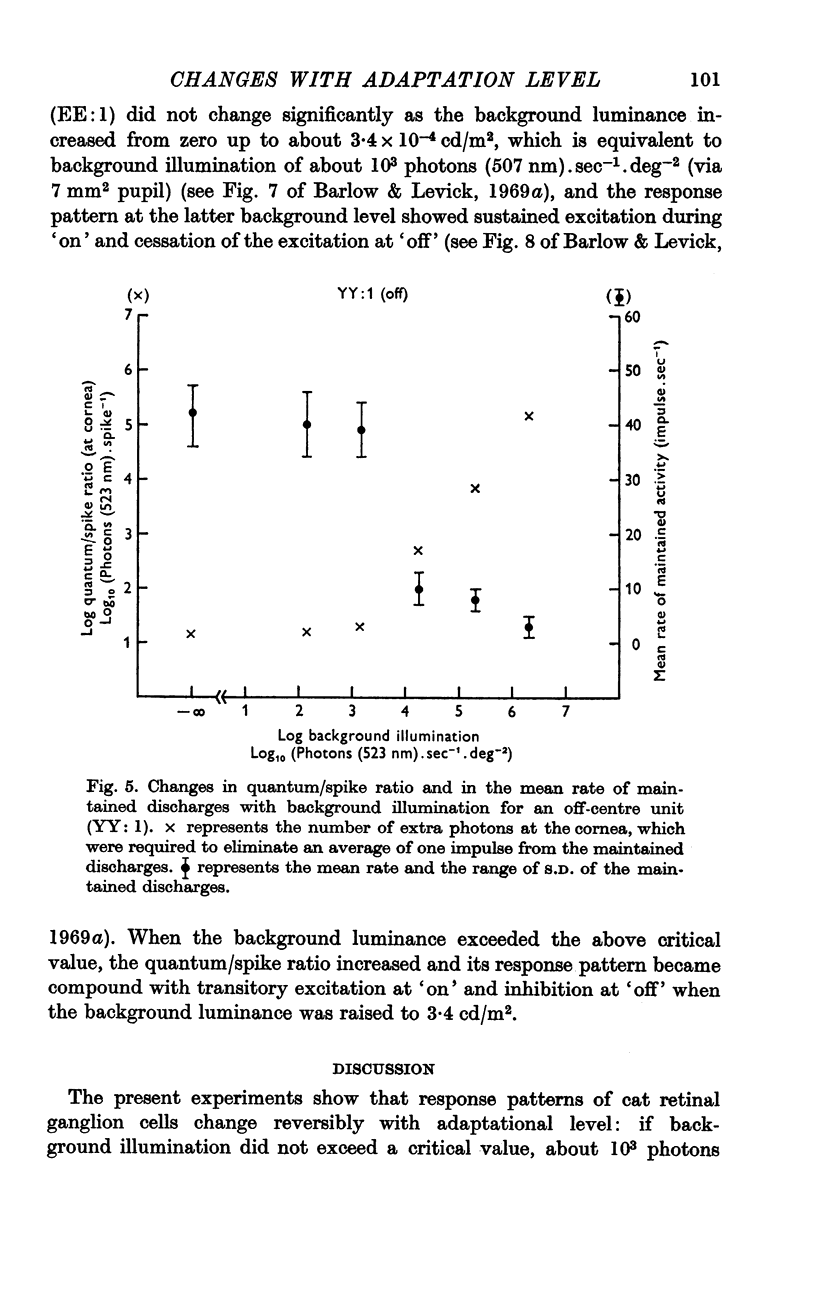
2. If background illumination did not exceed a critical value, about 103 photons (523 nm).sec-1.deg-2 (via 5·7 mm2 pupil), stimulation of the centre of a receptive field resulted in either sustained excitation (i.e. increase in discharge rate) during `on' and cessation of the excitation at `off' (on-centre unit), or sustained inhibition (i.e. decrease in discharge rate) during `on' and cessation of the inhibition at `off' (off-centre unit). Within this low adaptational level, a ganglion cell maintained its maximum sensitivity regardless of whether the weak background light was presented or not.
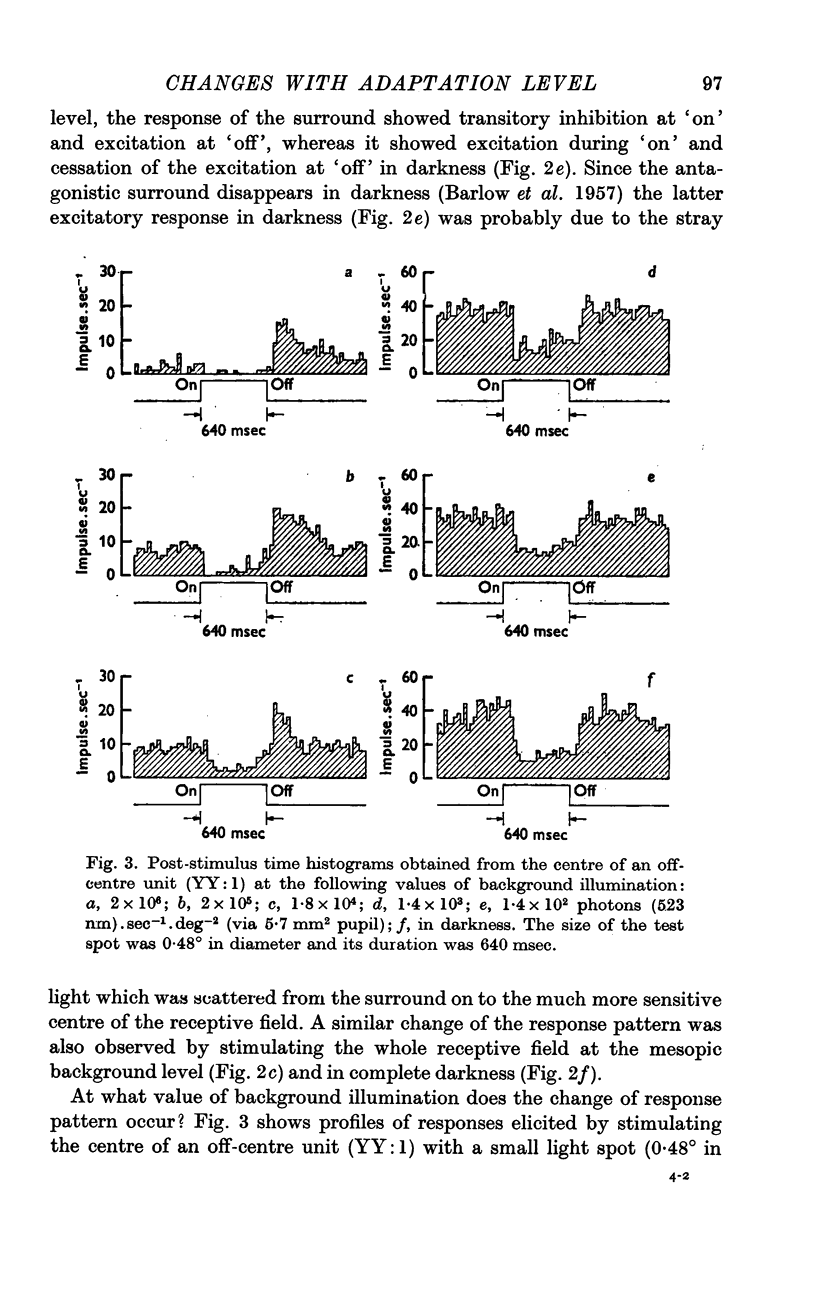
3. When background level exceeded the critical value up to 2 × 106 photons (523 nm).sec-1.deg-2, however, the simple, sustained responses changed into compound responses with two transient components of opposite polarities, either excitation at `on' and inhibition at `off' (on-centre unit), or inhibition at `on' and excitation at `off' (off-centre unit), and also the sensitivity began to decrease as the background increased, approximately obeying Weber's law.
4. It is suggested that a ganglion cell gives simple-sustained response when its gain control mechanism remains inactive at a low background illumination below a critical level, whereas it gives compound-transient response when its gain control mechanism becomes active as background illumination exceeds the critical value.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Matthews R. The action of light on the eye: Part I. The discharge of impulses in the optic nerve and its relation to the electric changes in the retina. J Physiol. 1927 Sep 9;63(4):378–414. doi: 10.1113/jphysiol.1927.sp002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Willmer E. N. An analysis of the response from single visual-purple-dependent elements, in the retina of the cat. J Physiol. 1950 Apr 15;111(1-2):160–173. doi: 10.1113/jphysiol.1950.sp004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. Stimulus intensity in relation to excitation and pre- and post-excitatory inhibition in isolated elements of mammalian retinae. J Physiol. 1944 Jun 15;103(1):103–118. doi: 10.1113/jphysiol.1944.sp004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R., Therman P. O. Excitation and inhibition in the retina and in the optic nerve. J Physiol. 1935 Feb 9;83(3):359–381. doi: 10.1113/jphysiol.1935.sp003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Purple R. L., Dodge F. A. Interaction of excitation and inhibition in the eccentric cell in the eye of Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:529–537. doi: 10.1101/sqb.1965.030.01.051. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Yoon M. Reversal of Weber's law for an extraordinary unit in the cat's retina. Vision Res. 1970 Aug;10(8):769–774. doi: 10.1016/0042-6989(70)90020-9. [DOI] [PubMed] [Google Scholar]


