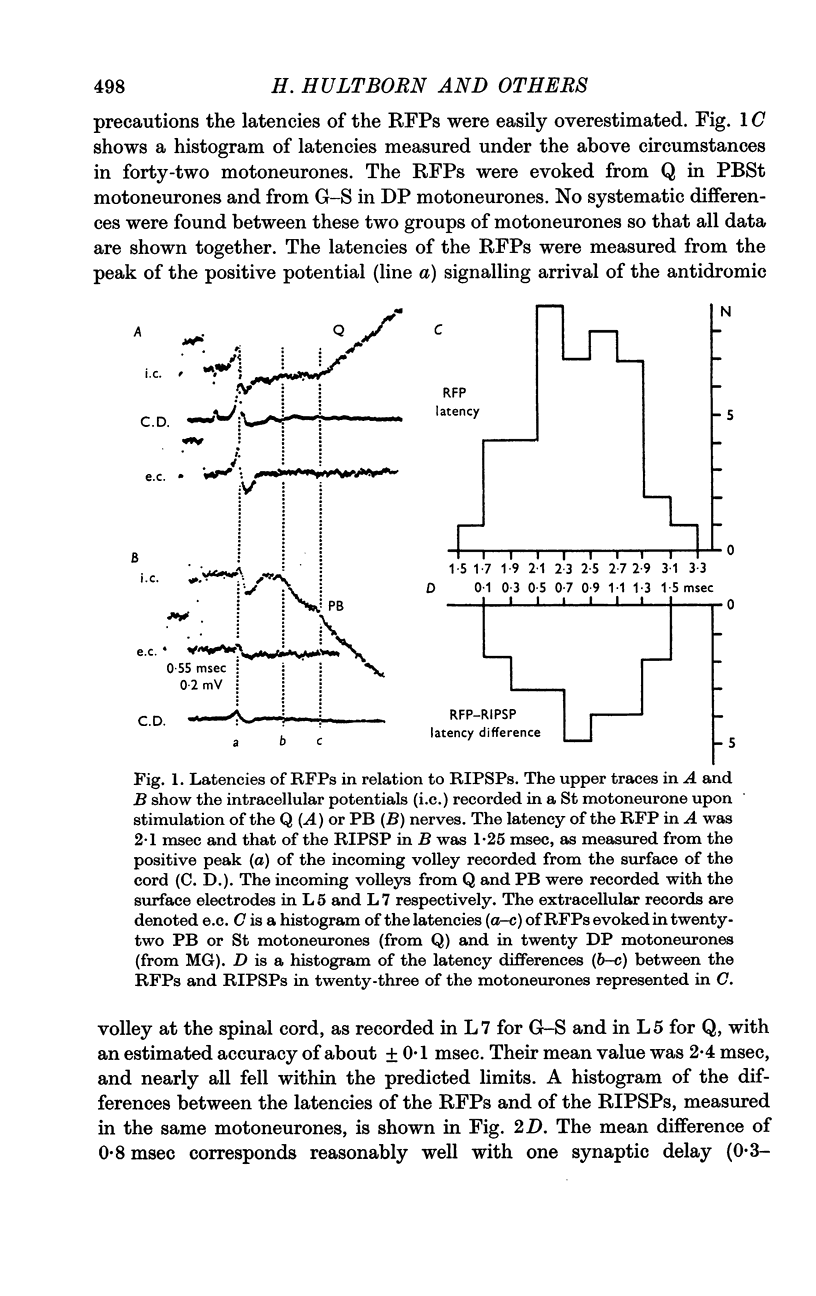
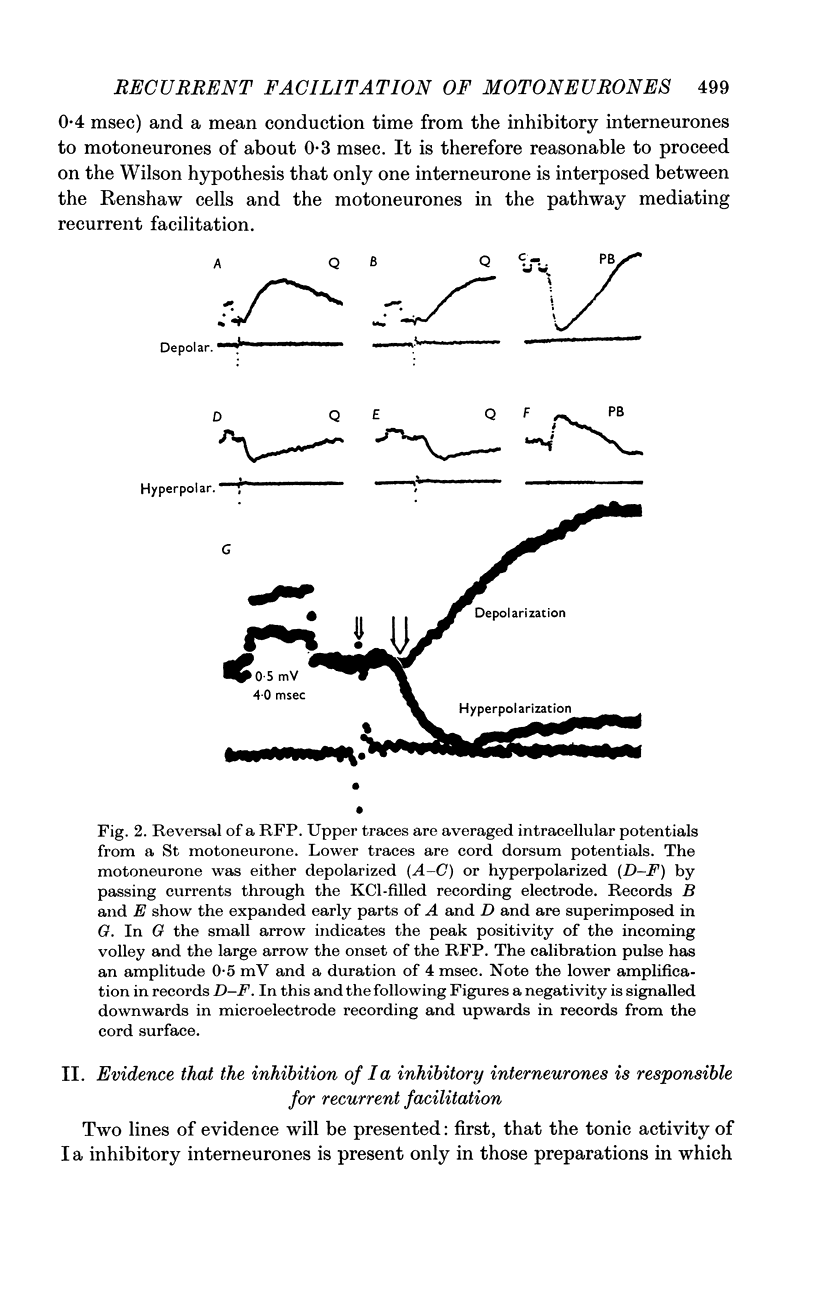
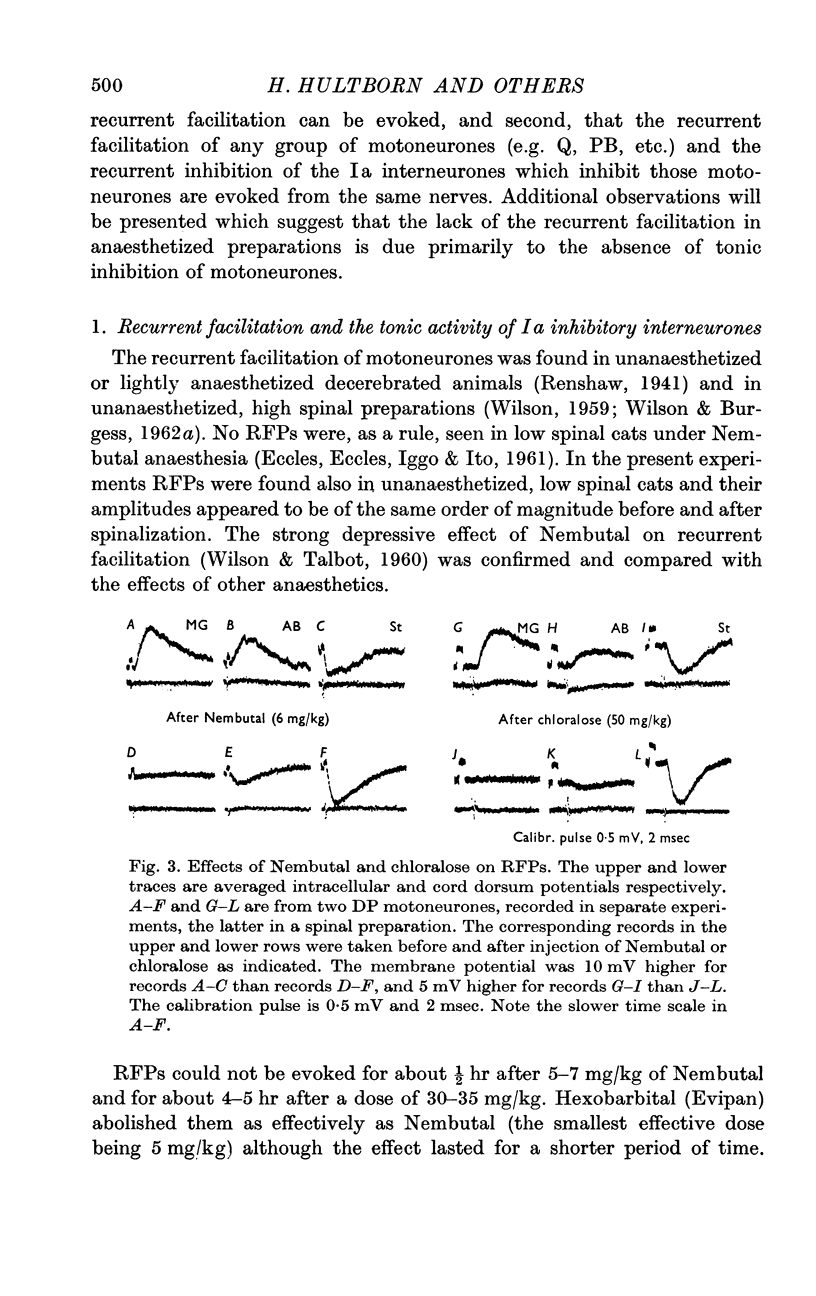
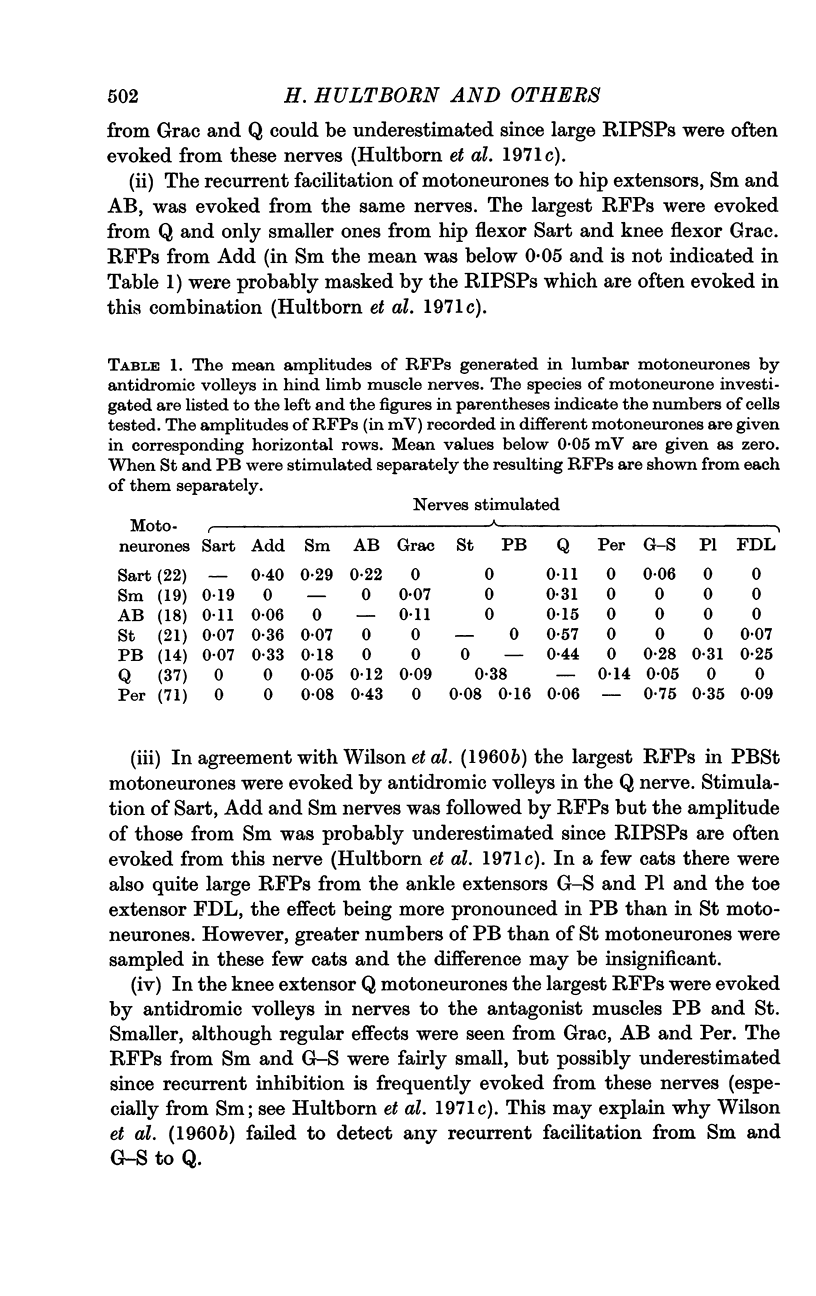
Abstract
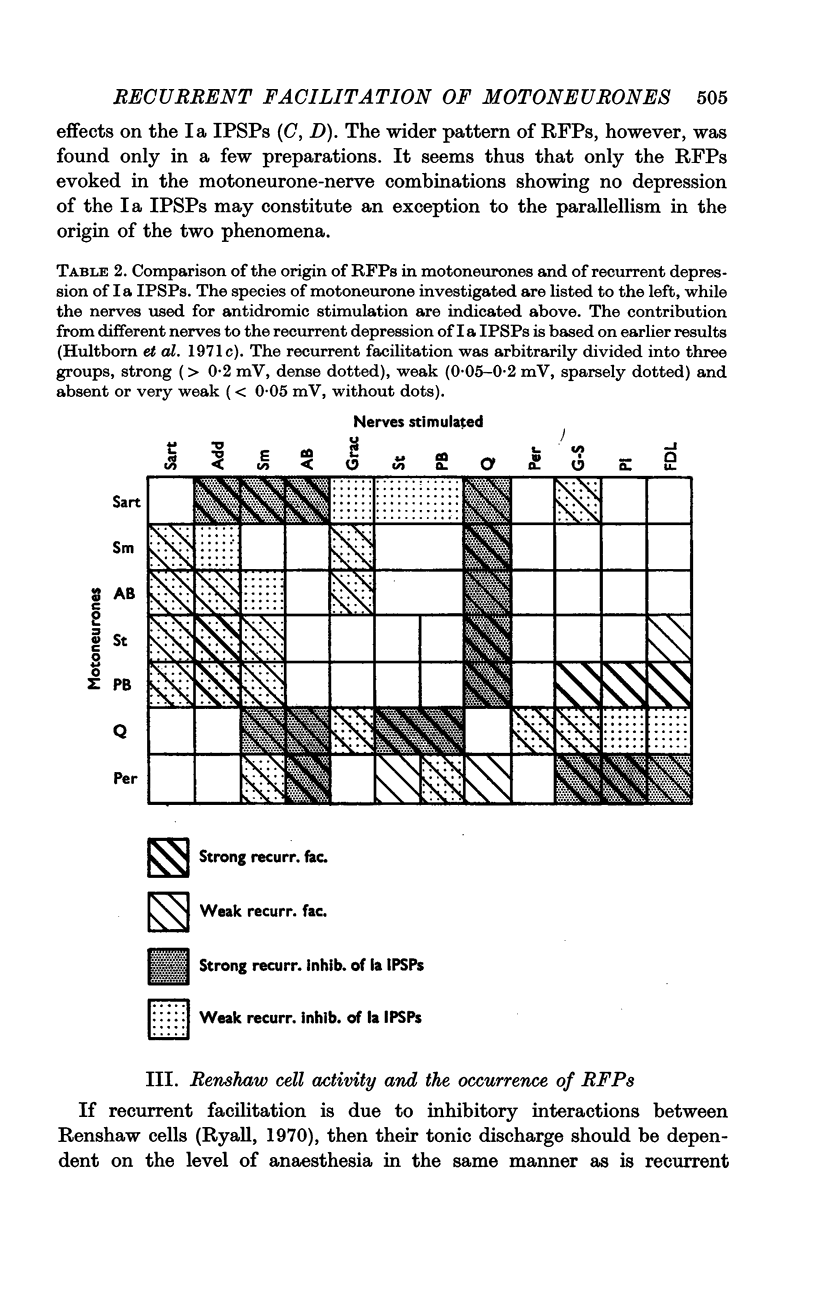
1. The recurrent facilitation of motoneurones is a disinhibition, i.e. a release of the motoneurones from a sustained hyperpolarization evoked by tonically active inhibitory interneurones. Only two groups of interneurones are known to receive recurrent inhibition from motor axon collaterals via Renshaw cells; the interneurones mediating the reciprocal Ia inhibition and the Renshaw cells themselves. The properties of these two groups of neurones were studied to determine if they could produce the tonic inhibition of motoneurones removed during recurrent facilitation.
2. It was found that the tonic firing of Ia inhibitory interneurones is sensitive to anaesthetics to the same degree as is recurrent facilitation. The range of frequencies of tonic discharges of Renshaw cells appeared to be similarly low in unanaesthetized and anaesthetized preparations although in individual cells the discharge rates were decreased by anaesthesia.
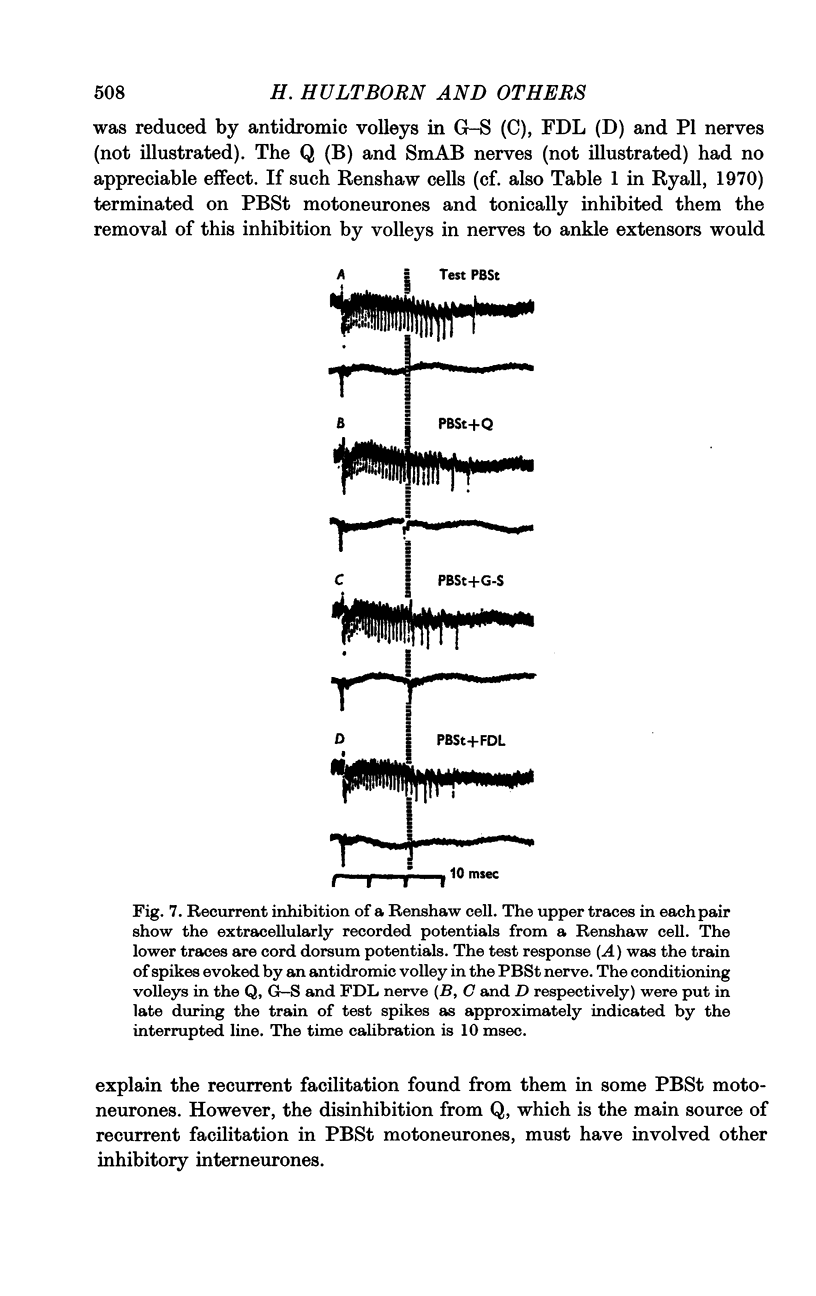
3. The recurrent inhibition of Ia interneurones inhibiting a given group of motoneurones and the recurrent facilitation of the same group of motoneurones were, as a rule, evoked from the same nerves, although in some cats the origin of the recurrent facilitation was somewhat wider. In contrast no evidence could be found that the Renshaw cells which inhibit a functional group of motoneurones are inhibited by volleys in the nerves from which recurrent facilitation is regularly evoked.
4. It was concluded that the recurrent facilitation is caused mainly by inhibition of the tonic activity of Ia inhibitory interneurones and that it is thus a manifestation of the recurrent control of Ia reciprocal inhibition of motoneurones.
Full text
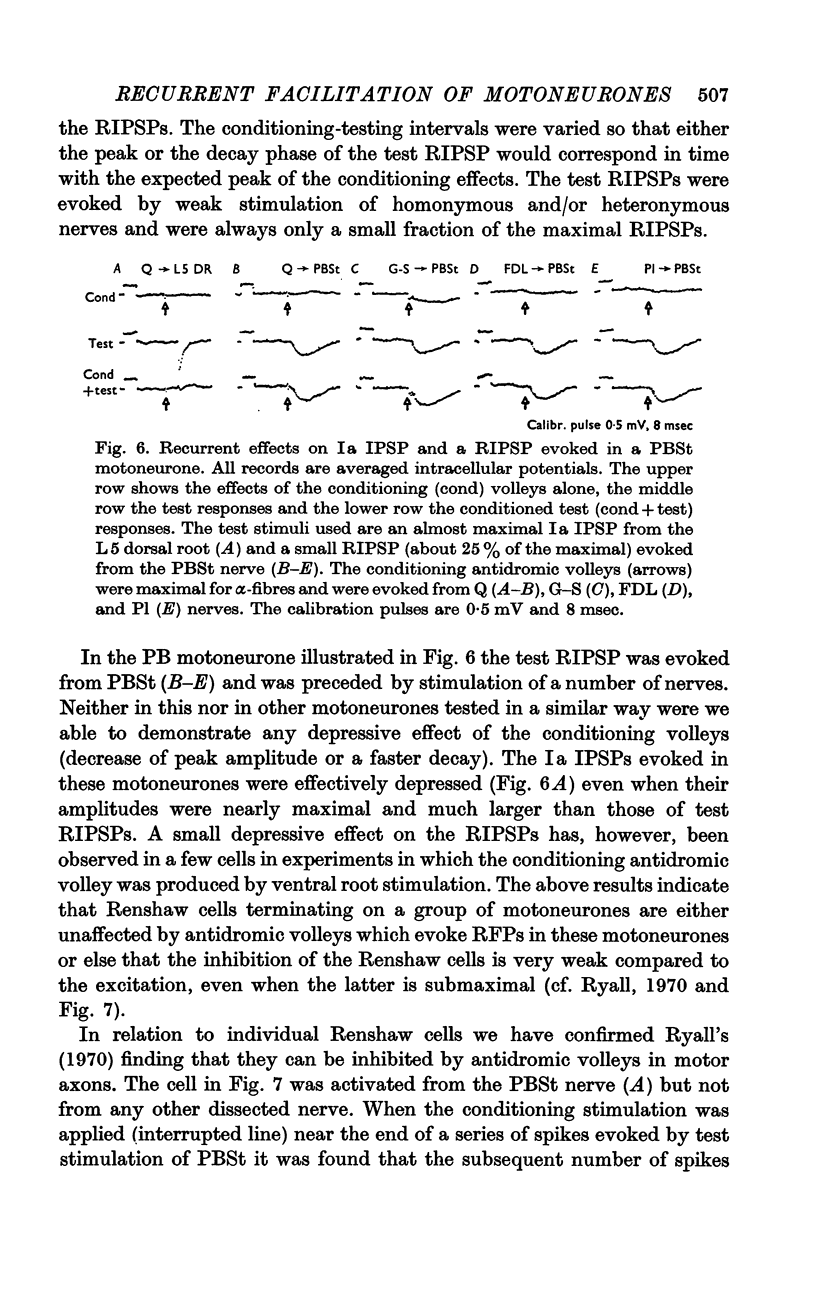
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966;2(1):81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961 Dec;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAASE J., van der MEULEN J. [The specific effect of chloralose on recurrent inhibition of tonic motor neurons]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;274:272–280. doi: 10.1007/BF00362318. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Inhibition in IA inhibitory pathway by impulses in recurrent motor axon collaterals. Life Sci. 1968 Apr 1;7(7):337–339. doi: 10.1016/0024-3205(68)90001-5. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Smerdlov S. M., Maksimova E. V. Effects of antidromic impulses on spontaneous activity of interneurons in the cat spinal cord. Fed Proc Transl Suppl. 1966 May-Jun;25(3):419–422. [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Effects of antidromic conditioning on some motoneurons and interneurons. J Neurophysiol. 1962 Sep;25:636–650. doi: 10.1152/jn.1962.25.5.636. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., DIECKE F. P., TALBOT W. H. Action of tetanus toxin on conditioning of spinal motoneurons. J Neurophysiol. 1960 Nov;23:659–666. doi: 10.1152/jn.1960.23.6.659. [DOI] [PubMed] [Google Scholar]
- WILSON V. J. Recurrent facilitation of spinal reflexes. J Gen Physiol. 1959 Mar 20;42(4):703–713. doi: 10.1085/jgp.42.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Distribution of recurrent facilitation and inhibition in cat spinal cord. J Neurophysiol. 1960 Mar;23:144–153. doi: 10.1152/jn.1960.23.2.144. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H. Recurrent conditioning in the cat spinal cord. Differential effect of meprobamate on recurrent facilitation and inhibition. J Gen Physiol. 1960 Jan;43:495–502. doi: 10.1085/jgp.43.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


