Abstract
1. Arterial blood samples were obtained from anaesthetized dogs in which either a respiratory alkalaemia, a respiratory acidaemia or a non-respiratory acidaemia was produced and from animals in which no deliberate changes in acid—base balance were produced. The samples were analysed for pH, carbon dioxide tension and concentration, oxygen tension and concentration and haematocrit. From these determinations the plasma carbon dioxide concentration, the plasma bicarbonate ion concentration and pK′ were calculated.
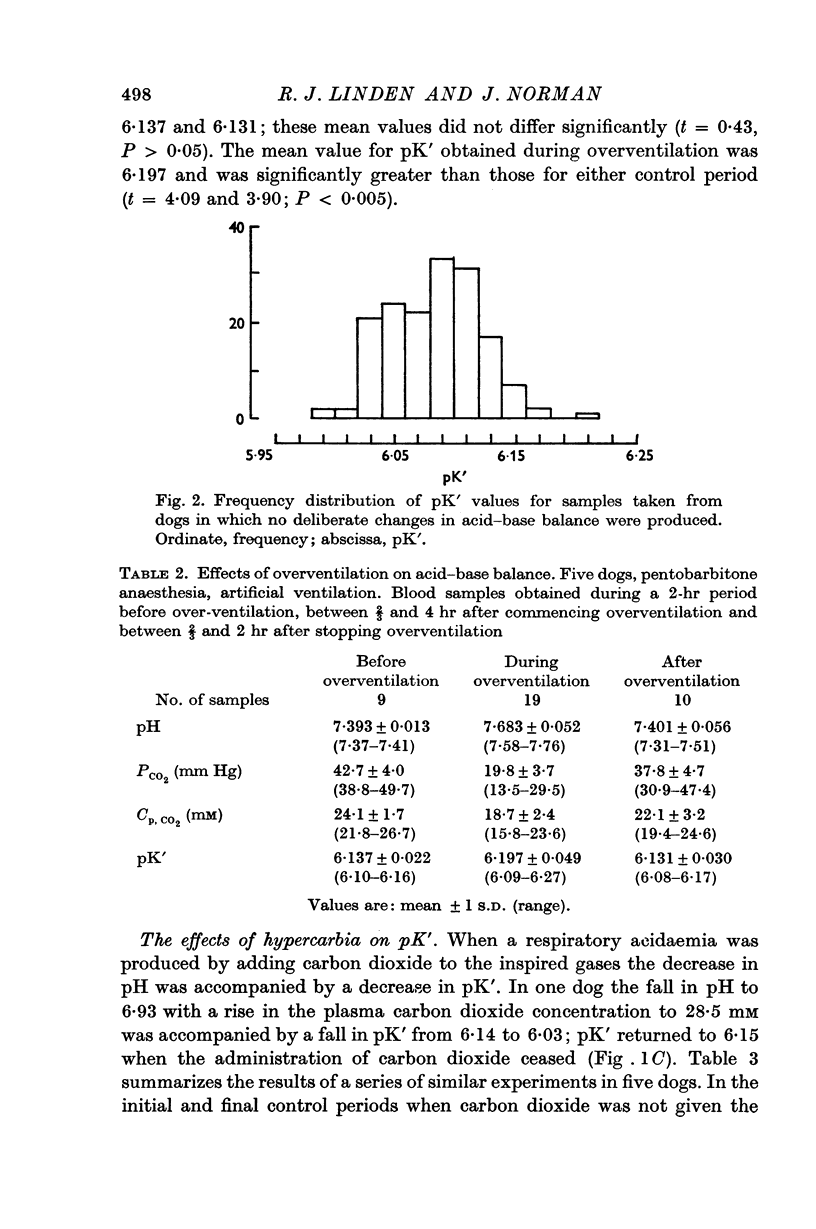
2. The value of pK′ lay within the range 5·86-6·27. The temperatures of the animals studied were between 36·75 and 39·25° C but there was no significant relationship between pK′ and temperature.
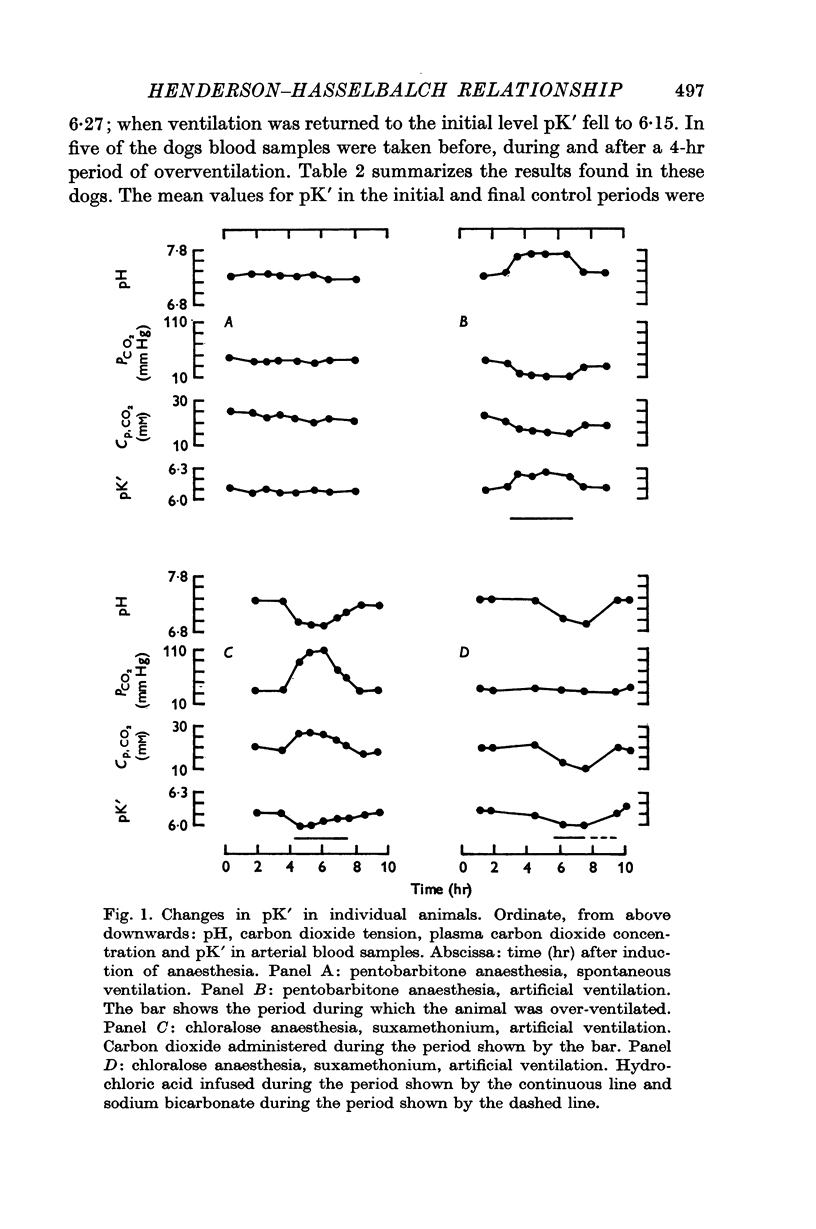
3. The values of pK′ increased with an alkalaemia and decreased with an acidaemia.
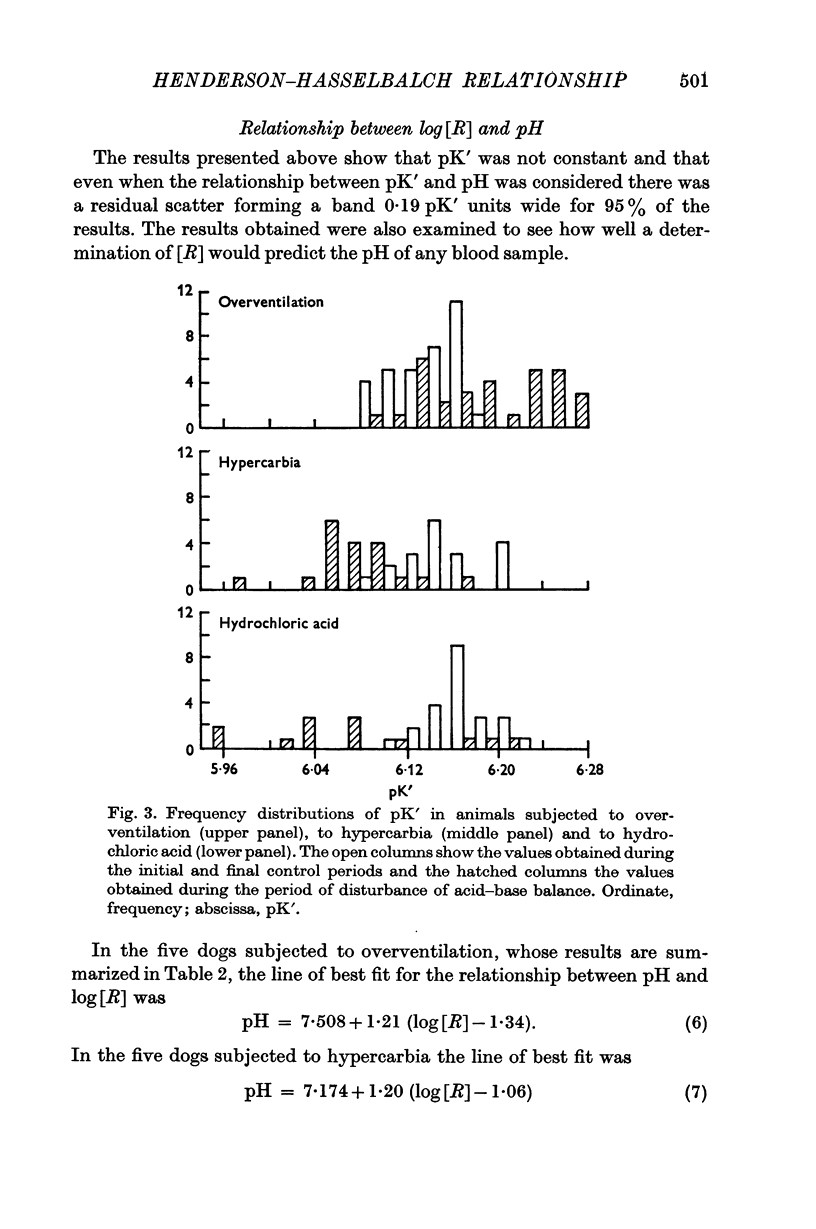
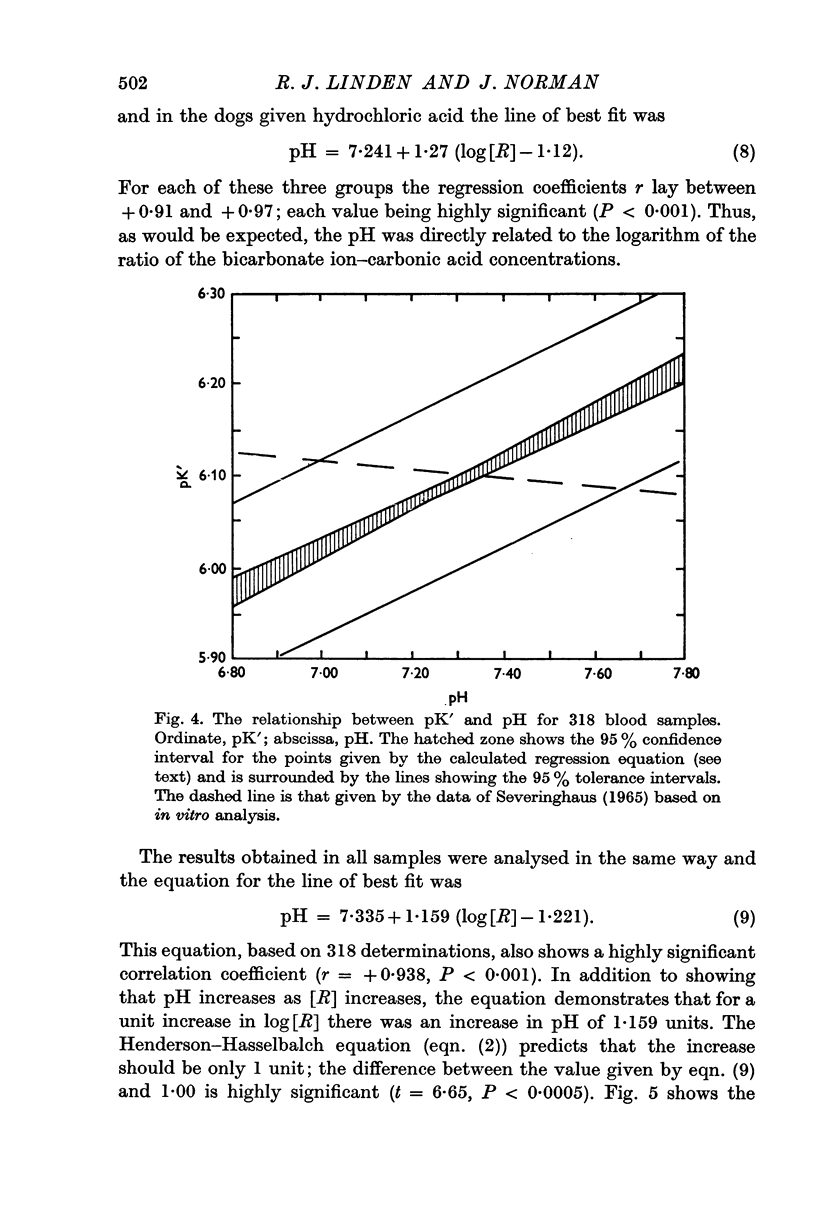
4. In addition to the variation of pK′ with pH there was a considerable residual scatter of pK′ values at any one pH value.
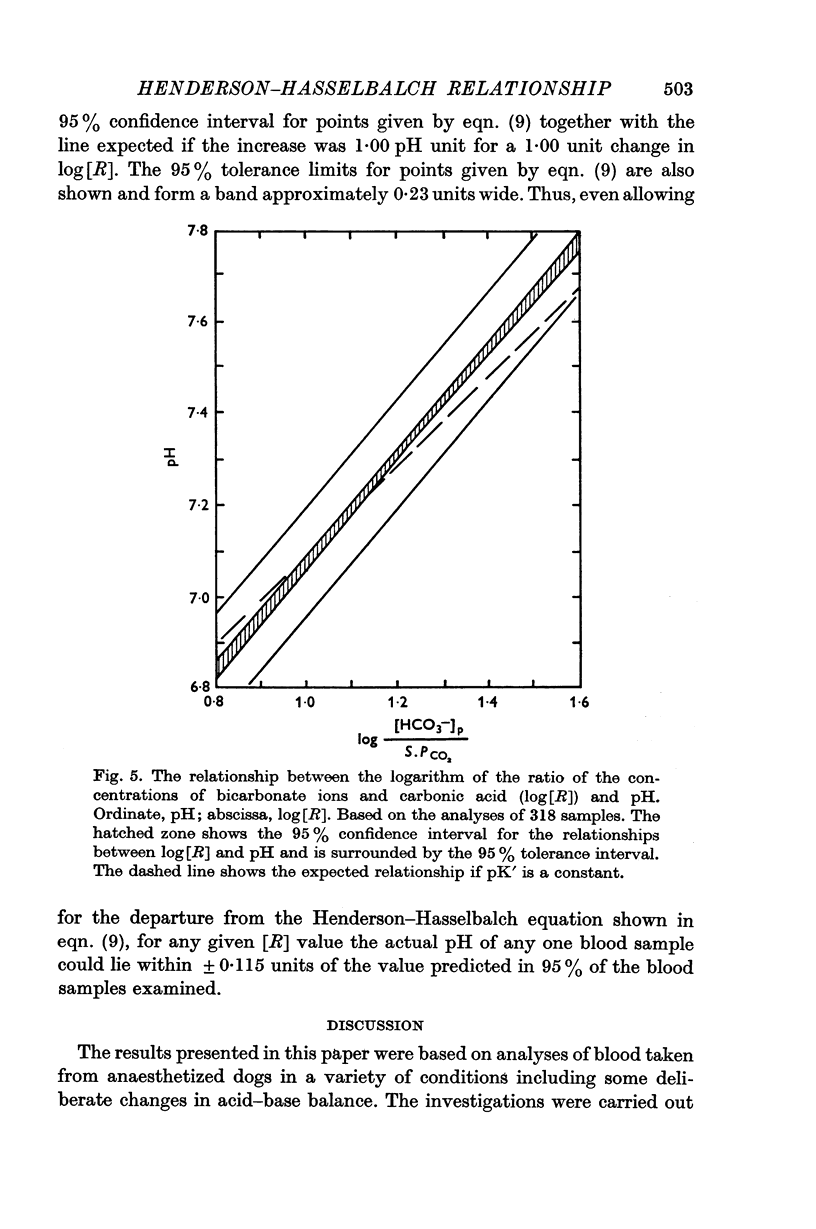
5. It was concluded that in these experimental conditions the use of the Henderson—Hasselbalch equation in a form not dealing with activities was unacceptable and could lead to much larger errors than has hitherto been thought.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN O. S. The first dissociation exponent of carbonic acid as a function of pH. Scand J Clin Lab Invest. 1962;14:587–597. [PubMed] [Google Scholar]
- AUSTIN W. H., LACOMBE E., RAND P. W., CHATTERJEE M. Solubility of carbon dioxide in serum from 15 to 38 C. J Appl Physiol. 1963 Mar;18:301–304. doi: 10.1152/jappl.1963.18.2.301. [DOI] [PubMed] [Google Scholar]
- BRADLEY A. F., SEVERINGHAUS J. W., STUPFEL M. Effect of temperature on PCO2 and PO2 of blood in vitro. J Appl Physiol. 1956 Sep;9(2):201–204. doi: 10.1152/jappl.1956.9.2.201. [DOI] [PubMed] [Google Scholar]
- BRADLEY A. F., SEVERINGHAUS J. W., STUPFEL M. Variations of serum carbonic acid pK with pH and temperature. J Appl Physiol. 1956 Sep;9(2):197–200. doi: 10.1152/jappl.1956.9.2.197. [DOI] [PubMed] [Google Scholar]
- BREWIN E. G., GOULD R. P., NASHAT F. S., NEIL E. An investigation of problems of acid base equilibrium in hypothermia. Guys Hosp Rep. 1955;104(3):177–214. [PubMed] [Google Scholar]
- LEDSOME J. R., LINDEN R. J., NORMAN J. THE USE OF SYMPATHETIC BETA-RECEPTOR BLOCKING AGENTS IN THE INVESTIGATION OF REFLEX CHANGES IN HEART RATE. Br J Pharmacol Chemother. 1965 Jun;24:781–788. doi: 10.1111/j.1476-5381.1965.tb01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEN R. J., LEDSOME J. R., NORMAN J. SIMPLE METHODS FOR THE DETERMINATION OF THE CONCENTRATIONS OF CARBON DIOXIDE AND OXYGEN IN BLOOD. Br J Anaesth. 1965 Feb;37:77–88. doi: 10.1093/bja/37.2.77. [DOI] [PubMed] [Google Scholar]
- Ledsome J. R., Linden R. J., Norman J. The effect of light chloralose and pentobarbitone anaesthesia on the acid-base state and oxygenation of arterial blood in dogs. J Physiol. 1971 Feb;212(3):611–627. doi: 10.1113/jphysiol.1971.sp009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsome J. R., Linden R. J. The effect of distending a pouch of the left atrium on the heart rate. J Physiol. 1967 Nov;193(1):121–129. doi: 10.1113/jphysiol.1967.sp008346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R. J., Norman J. The effect of acidaemia on the response to stimulation of the autonomic nerves to the heart. J Physiol. 1969 Jan;200(1):51–71. doi: 10.1113/jphysiol.1969.sp008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J., Ledsome J. R., Linden R. J. A system for the measurement of respiratory and acid-base parameters in blood. Br J Anaesth. 1965 Jul;37(7):466–479. doi: 10.1093/bja/37.7.466. [DOI] [PubMed] [Google Scholar]
- Trenchard D., Noble M. I., Guz A. Serum carbonic acid pK'1 abnormalities in patients with acid-base disturbances. Clin Sci. 1967 Apr;32(2):189–200. [PubMed] [Google Scholar]


