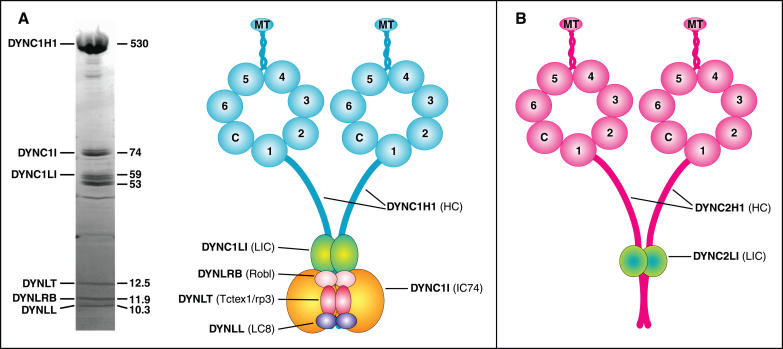
Figure 1. The Mammalian Cytoplasmic Dynein Complexes.
(A) Cytoplasmic dynein. (Left panel) Polypeptides of immunoaffinity-purified rat brain cytoplasmic dynein. Polypeptide mass (in kDa) is indicated on the right side of the gel, and the consensus family names are indicated on the left. (Right panel) Structural model for the association of the cytoplasmic dynein complex subunits. The core of the cytoplasmic dynein complex is made of two DYNC1H1 heavy chains which homodimerize via regions in their N-termini. The motor domains are at the C-termini of the heavy chains, the large globular heads of ~350 kDa that are composed of a ring of seven densities surrounding a central cavity; six of the densities are AAA domains (numbered 1–6). AAA domain 1 is the site of ATP hydrolysis. The microtubule-binding domain is a projection found on the opposite side of the ring between AAA domains 4 and 5. C is the C-terminus of the heavy chain that would form the 7th density. Two DYNC1I intermediate chains (IC74) and DYNC1LI light intermediate chains bind at overlapping regions of the N-terminus of the heavy chain, overlapping with the heavy chain dimerization domains. Dimers of the three light chain families; DYNLT, the Tctex1 light chains; DYNLRB, the Roadblock light chains; and DYNLL, the LC8 light chains, bind to the intermediate chain dimers.
(B) Cytoplasmic dynein 2 complex, structural model for subunit association. This dynein complex has a unique role in IFT and is sometimes known as IFT dynein. Structural predictions indicate that the heavy chain, DYNC2H1, is similar to the cytoplasmic and axonemal dyneins. The only known subunit of this complex is a 33- to 47-kDa polypeptide, DYNC2LI1, which is related to the cytoplasmic dynein light intermediate chains. No intermediate chain or light chains have yet been identified [16].