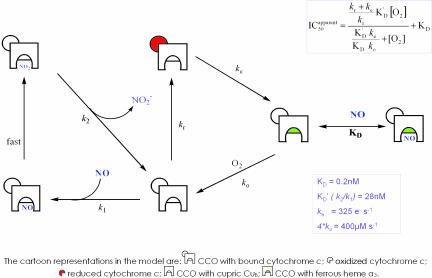
Fig. 3.
A theoretical model that can explain NO inhibition under all conditions of enzyme turnover and oxygen tension. In this model NO can interact both competitively with oxygen and noncompetitively, reacting with the binuclear center in either the reduced (ferrous a3) or oxidized (cupric CuB) state. The equation for IC50 is a rectangular hyperbola, in which the upper value of ICapparent50 is related to turnover (kr) (see boxed equation), and KD′ = k2/k1. The fitted values of KD and KD′ were 0.2 nM and 28 nM, respectively. The rate of reaction with oxygen (ko) is 4 * 100 μM–1, where the factor 4 appears because four electrons are moved to oxygen per reaction step. The internal electron transfer rate ke is set at 325 s–1. Values of kr (initial reduction rate of the binuclear center) are set to accord with the measured turnovers (Fig. 2). Note that, although a number of iron:oxygen intermediates (e.g., ferryl) are also likely to be present during turnover, the interactions of NO with these oxidized species (26, 27) are formally included in the reaction step governed by k1, and so this added complexity does not affect the conclusions drawn from this model. Additionally, the reaction step governed by k2 is more complex than indicated because the nitrite dissociation rate depends on the redox state of the binuclear center (29). Thus, KD′ represents an apparent, not true, affinity constant. (Note that cytochrome c is illustrated bound to CCO to denote the source of electrons, but this does not imply cytochrome c remains bound throughout multiple catalytic cycles.)