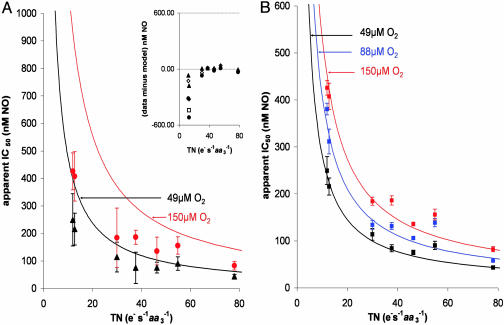
Fig. 4.
Apparent IC50 for NO as a function of enzyme turnover at different [O2]. (A) Points represent experimental data, error bars represent 95% confidence limit, and solid lines are theoretical fits obtained by using the model and associated equation proposed by Antunes et al. (43): IC50 = Ki (1 + kO2app/kiv [O2]), where Ki = 0.2 nM, kiv is the turnover number in units of e– s–1aa3–1, and kO2app was calculated according to Verkhovsky et al. (53). (Inset) the residual obtained after subtracting the theoretical fits from the actual data (•, 150 μM O2; □, 122 μM O2; ⋄, 88 μM O2; ▴, 49 μM O2). (B) The same data points with solid fit lines corresponding to our model; equation and values are shown in Fig. 3 and its legend (error bars represent SEM). A model in which NO interacts with the binuclear center in both oxidized and reduced states clearly fits the data more closely than does the simple competitive model.