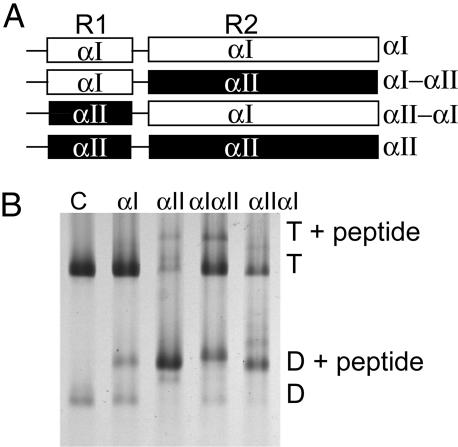
Fig. 4.
Binding of αI- and αII-spectrin fragments to spectrin dimer self-association sites in ghost membranes at 37°C. (A) Schematic diagram of α-spectrin fragments. R1 is the partial triple helical repeat that binds to β.R2 is the first full triple helix. αIαII and αIIαI are hybrids in which R1 and R2 are swapped between the proteins. (B) Spectrin extracted from the resealed ghosts was analyzed by electrophoresis in 5% nondenaturing gels. Incorporation at 100 μM total concentration of each fragment was demonstrated by the presence of a new band, migrating above the spectrin dimer (D) and tetramer (T). Lane 1, control spectrin with no peptide introduced into the ghosts; lane 2, αI fragment incorporated; lane 3, αII fragment incorporation; lane 4, αIαII; lane 5 αIIαI.