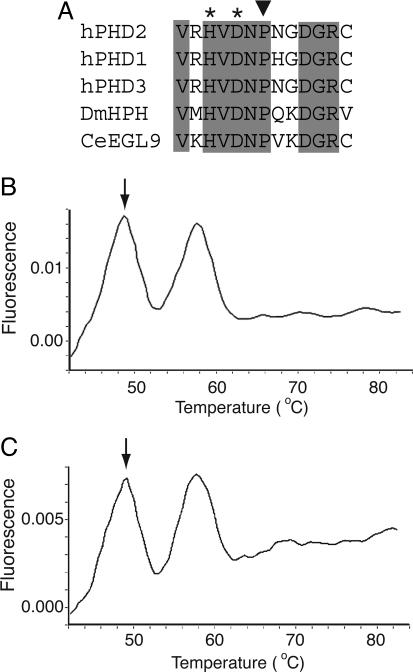
Fig. 1.
The 950 C → G mutation affects a residue in the active site of PHD2. (A) Comparison of amino acid sequence from residues 311–323 (human PHD2 numbering) in human HIF prolyl hydroxylases as well as those from Drosophila melanogaster (DmHPH) and Caenorhabditis elegans (CeEGL9). Shading indicates completely conserved residues, asterisks indicate iron-chelating residues, and the inverted triangle indicates Pro-317 of human PHD2, which is predicted to be changed to Arg by the 950 C → G mutation. (B and C) Detection of 950 C → G PHD2 mutation by a hybridization probe. By using a fluorescently labeled hybridization probe specific to the normal sequence, the two heterozygous erythrocytosis siblings were screened with the Roche Diagnostics LightCycler. The annealing temperature of the probe was 58°C when hybridized to the normal sequence. The mismatch due to the presence of the 950 C → G PHD2 mutation reduced the annealing temperature to 49°C (arrows). Both siblings exhibited two peaks, indicating the presence of both the normal and mutant sequences.