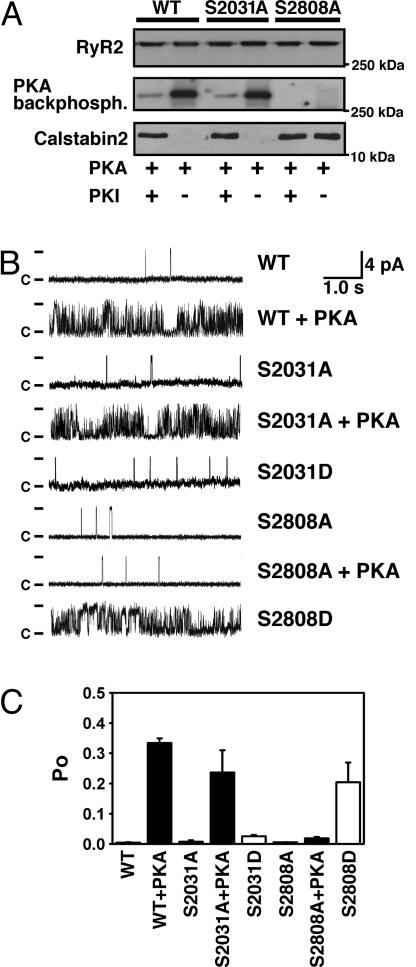
Fig. 2.
PKA phosphorylation of Ser-2808 but not Ser-2031 modulates RyR2 single-channel activity. (A) Representative WT and mutant recombinant human RyR2 channels, coexpressed with calstabin2 (FKBP12.6), were PKA-phosphorylated in the presence or absence of the PKA-inhibitor PKI5–24. Alanine substitution of Ser-2808, but not Ser-2031, prevents PKA phosphorylation of RyR2. Moreover, PKA phosphorylation of WT and RyR2-S2031A channel reduced the binding affinity of the RyR2 subunit calstabin2, whereas calstabin2 did not dissociate from RyR2-S2808A channels treated with PKA. (B) Single-channel recordings of RyR2-WT and mutant RyR2. PKA phosphorylation of RyR2-WT and RyR2-S2031A channels increased Po at low-cytosolic Ca2+ concentrations (150 nM), whereas the mutant RyR2-S2808A channels did not exhibit PKA phosphorylation-induced increase in Po. The Asp substitution of Ser-2031 functionally mimicked PKA phosphorylation of this residue but did not cause an increase in Po of the RyR2-S2031D channels. In contrast, RyR2-S2808D channels mimicked constitutively PKA-phosphorylated RyR2-WT channels, confirming that Ser-2808 is the only functional PKA site on RyR2. (C) Summary values of Po of single-channel recordings described in B (n = 4–9 channels in each group).