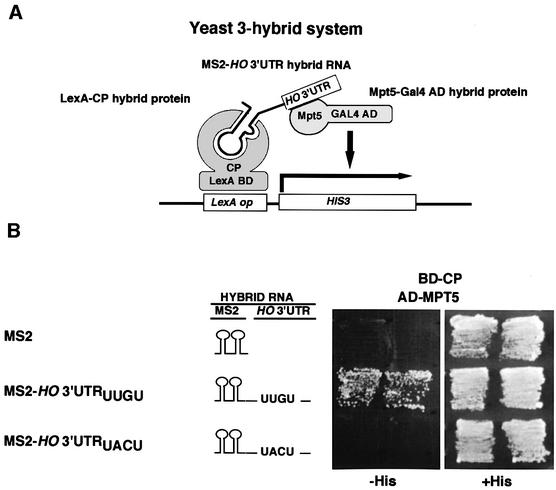
Fig. 6. Requirement for the HO mRNA 3′-UTR for functioning of Mpt5 in the three-hybrid RNA-binding assay. (A) Schematic drawing of the yeast three-hybrid assay. The LexA-CP hybrid protein (BD-CP) contains the LexA DNA-binding domain joined to the MS2 coat protein and binds to the LexA operator of the HIS3 reporter gene. The Gal4 AD-Mpt5 hybrid protein (AD-MPT5) is a fusion of Mpt5 and the yeast Gal4 activation domain. The hybrid RNA (MS2-HO 3′-UTR) contains the binding site for MS2 coat protein and the HO 3′-UTR. If the Mpt5 segment of the Gal4 AD-Mpt5 hybrid binds to the HO 3′-UTR, then the MS2-HO 3′-UTR hybrid RNA will link the two hybrid proteins to each other and activate the HIS3 reporter gene. (B) Yeast strain L40c, which expresses a LexA-MS2 coat protein fusion, was transformed with the indicated plasmids, and the transformants were streaked on SC-Ura Leu His (–His) and SC-Ura Leu (+His) plates and incubated for 2 (+His plates) or 3 days (–His plates) at 30°C. Plasmids were MS2, MS2-HO 3′-UTRUUGU, MS2-HO 3′-UTRUACU and pGAD-MPT5.
